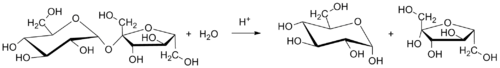Inverted sugar syrup
 | |
| Identifiers | |
|---|---|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.029.446 |
PubChem CID |
|
| Properties | |
| Molar mass | 360.312 g/mol |
| Pharmacology | |
| C05BB03 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Invert(ed)[1] sugar (syrup) is an edible mixture of two simple sugars—glucose and fructose—that is made by heating sucrose (table sugar) with water. Invert sugar is thought to be sweeter than table sugar,[2][3] and foods that contain it retain moisture and crystallize less easily. Bakers, who call it invert syrup, may use it more than other sweeteners.[4]
Though invert sugar syrup can be made by heating table sugar in water alone, the reaction can be sped up by adding lemon juice, cream of tartar or other catalysts often without changing the flavor noticeably.
The mixture of the two simple sugars is formed by a process of hydrolysis of sucrose. This mixture has the opposite direction of optical rotation as the original sugar, which is why it is called an invert sugar.
Chemistry
Table sugar (sucrose) is converted to invert sugar by a chemical reaction called hydrolysis. Heating a mixture or solution of table sugar and water breaks the chemical bond that links together the two simple-sugar components.
The balanced chemical equation for the hydrolysis of sucrose into glucose and fructose is:
- C12H22O11 (sucrose) + H2O (water) → C6H12O6 (glucose) + C6H12O6 (fructose)
Optical rotation
Once a sucrose solution has had some of its sucrose turned into glucose and fructose the solution is no longer said to be pure. The gradual decrease in purity of a sucrose solution as it is hydrolyzed affects a chemical property of the solution called optical rotation that can be used to figure out how much of the sucrose has been hydrolyzed and therefore whether the solution has been inverted or not.
Definition and measurement
A kind of light called plane polarized light can be shone through a sucrose solution as it is heated up for hydrolysis. Such light has an 'angle' that can be measured using a tool called a polarimeter. When such light is shone through a solution of pure sucrose it comes out the other side with a different angle than when it entered; its angle is therefore said to be 'rotated' and how many degrees the angle has changed (the degree of its rotation or its 'optical rotation') is given a letter name, (alpha). When the rotation between the angle the light has when it enters and when it exits is in the clockwise direction, the light is said to be 'rotated right' and is given to have a positive angle like . When the rotation between the angle the light has when it enters and when it exits is in the counterclockwise direction, the light is said to be 'rotated left' and is given a negative angle like .
Definition of the inversion point
When plane polarized light enters and exits a solution of pure sucrose its angle is rotated (clockwise or to the right). As the sucrose is heated up and hydrolyzed the amount of glucose and fructose in the mixture increases and the optical rotation decreases. After passes zero and becomes a negative optical rotation, meaning that the rotation between the angle the light has when it enters and when it exits is in the counter clockwise direction, it is said that the optical rotation has 'inverted' its direction. This leads to the definition of an 'inversion point' as the per cent amount sucrose that has to be hydrolyzed before equals zero. Any solution which has passed the inversion point (and therefore has a negative value of ) is said to be 'inverted'.
Chirality and specific rotation
As the shapes of the molecules ('chemical structures') of sucrose, glucose and fructose are all asymmetrical the three sugars come in several different forms, called stereoisomers. The existence of these forms is what gives rise to these chemicals' optical properties. When plane polarized light passes through a pure solution of one of these forms of one of the sugars it is thought to hit and 'glance off' certain asymmetrical chemical bonds within the molecule of that form of that sugar. Because those particular bonds (which in cyclic sugars like sucrose, glucose and fructose include a kind of bond called an anomeric bond) are different in each form of the sugar, each form rotates the light to a different degree.
When any one form of a sugar is purified and put in water, it rapidly takes other forms of the same sugar. This means that a solution of a pure sugar normally has all of its stereoisomers present in the solution in different amounts which usually do not change much. This has an 'averaging' effect on all of the optical rotation angles ( values) of the different forms of the sugar and leads to the pure sugar solution having its own 'total' optical rotation, which is called its 'specific rotation' or 'observed specific rotation' and which is written as .
| Specific optical rotations of pure sugars | |
|---|---|
| Sugar | (degrees) |
| sucrose | (+) 66.5 |
| glucose | (+) 52.7 |
| fructose | (-) 92.0 |
Effects of water
Water molecules do not have chirality, therefore they do not have any effect on the measurement of optical rotation. When plane polarized light enters a body of pure water its angle is no different than when it exits. Thus, for water, . Chemicals that, like water, have specific rotations that equal zero degrees are called 'optically inactive' chemicals and like water, they do not need to be considered when calculating optical rotation.
Mixtures in general
The overall optical rotation of a mixture of chemicals can be calculated if the proportion of the amount of each chemical in the solution is known. If there are -many optically active different chemicals ('chemical species') in a solution and the molar concentration (the number of moles of each chemical per Liter of liquid solution) of each chemical in the solution is known and written as (where is a number used to identify the chemical species); and if each species has a specific rotation (the optical rotation of that chemical were it made as a pure solution) written as , then the mixture has the overall optical rotation
Where is the mole fraction of the species.
Fully hydrolyzed sucrose
Assuming no extra chemical products are formed by accident (that is, there are no side reactions) a completely hydrolyzed sucrose solution no longer has any sucrose and is a half-and-half mixture of glucose and fructose. This solution has the optical rotation
Partly hydrolyzed sucrose
If a sucrose solution has been partly hydrolyzed, then it contains sucrose, glucose and fructose and its optical rotation angle depends on the relative amounts of each for the solution;
Where , , and stand for sucrose, glucose, and fructose. The particular values of do not need to be known to make use of this equation as the inversion point (per cent amount of sucrose that must be hydrolyzed before the solution is inverted) can be calculated from the specific rotation angles of the pure sugars. The reaction stoichiometry (the fact that hydrolyzing one sucrose molecule makes one glucose molecule and one fructose molecule) shows that when a solution begins with moles of sucrose and no glucose nor fructose and moles of sucrose are then hydrolyzed the resulting solution has moles of sucrose, moles of glucose and moles of fructose. The total number of moles of sugars in the solution is therefore and the reaction progress (per cent completion of the hydrolysis reaction) equals . It can be shown that the solution's optical rotation angle is a function of (explicitly depends on) this per cent reaction progress. When the quantity is written as and the reaction is done, the optical rotation angle is
By definition, equals zero degrees at the 'inversion point'; to find the inversion point, therefore, alpha is set equal to zero and the equation is manipulated to find . This gives
Thus it is found that a sucrose solution is inverted once at least of the sucrose has been hydrolyzed into glucose and fructose.
Monitoring reaction progress
Holding a sucrose solution at temperatures of 50–60 °C (122–140 °F) hydrolyzes no more than about 85 per cent of its sucrose. Finding when shows that the optical rotation of the solution after hydrolysis is done is ; this reaction is said to invert the sugar because its final optical rotation is less than zero. A polarimeter can be used to figure out when the inversion is done by detecting whether the optical rotation of the solution at an earlier time in its hydrolysis reaction equals .
Production
Common sugar can be inverted quickly by mixing sugar and citric acid or cream of tartar at a ratio of about 1000:1 by weight and adding water. If lemon juice which is about five per cent citric acid by weight is used instead then the ratio becomes 1:75. Such a mixture, heated to 114 °C (237 °F)[5] and added to another food, prevents crystallization without tasting sour.
Invert sugar syrup can be made without acids or enzymes by heating it up alone: two parts granulated sugar and one part water, simmered for five to seven minutes, will be partly inverted.
Basic formula
by weight
[note 1]sucrose 100% water 50% acid 0.1%
Commercially prepared enzyme-catalyzed solutions are inverted at 60 °C (140 °F). The optimum pH for inversion is 5.0. Invertase is added at a rate of about 0.15% of the syrup's weight, and inversion time will be about 8 hours. When completed the syrup temperature is raised to inactivate the invertase, but the syrup is concentrated in a vacuum evaporator to preserve color.[6]
Commercially prepared hydrochloric-acid catalysed solutions may be inverted at the relatively low temperature of 50 °C (122 °F). The optimum pH for acid-catalysed inversion is 2.15. As the inversion temperature is increased, the inversion time decreases.[6] They are neutralized when the desired level of inversion is reached.[7][8]
In confectionery and candy making, cream of tartar is commonly used as the acidulant, with typical amounts in the range of 0.15-0.25% of the sugar's weight.[9] The use of cream of tartar imparts a honey-like flavor to the syrup.[8] After the inversion is completed, it may be neutralized with baking soda using a weight of 45% of the cream of tartar's weight.[10][11]
Neutralization
[note 2]cream of tartar 100% baking soda 45%
When adding baking soda and whipping or mixing, the hot syrup will foam and bubble up, so some care is required. A much taller pan than otherwise needed will contain the foam. Make sure there is enough water remaining in the syrup to dissolve the baking soda. Alternatively, dissolve the baking soda in a little extra water, and ensure the syrup's temperature is somewhat below 100 °C (212 °F).[12]
Initial sucrose concentration and boiling point[13] Sucrose Water Boiling point 30% 70% 100 °C (212 °F) 40% 60% 101 °C (214 °F) 50% 50% 102 °C (216 °F) 60% 40% 103 °C (217 °F) 70% 30% 106 °C (223 °F) 80% 20% 112 °C (234 °F) 90% 10% 123 °C (253 °F) 95% 5% 140 °C (284 °F) 97% 3% 151 °C (304 °F) 98.2% 1.8% 160 °C (320 °F) 99.5% 0.5% 166 °C (331 °F) 99.6% 0.4% 171 °C (340 °F)
The amount of water can be increased to increase the time it takes to reach the desired final temperature, and increasing the time increases the amount of inversion that occurs.[12] In general, higher final temperatures result in thicker syrups, and lower final temperatures, in thinner ones.
All constituent sugars (sucrose, glucose and fructose) support fermentation, so invert sugar solutions may be fermented as readily as sucrose solutions.
Shelf life
Fully inverted sugar's low water content improves the shelf lives of products that contain it. The shelf life of partly inverted sugar is about six months and varies by storage and climatic conditions.
Crystallized invert sugar solutions can be restored to their liquid state by gently heating.
In other foods and products
- Honey which is mostly a mixture of glucose and fructose, being similar to invert syrup therefore, can remain a liquid for long periods of time.
- Jam contains invert sugar formed by the heating process and the acid content of the fruit. This sugar preserves the jam for long periods of time.
- Golden syrup is a syrup of about 55% invert syrup and 45% table sugar (sucrose).
- Fondant filling for chocolates is unique in that the conversion enzyme is added, but not activated by acidification (microenvironment pH adjustment) or cofactor addition depending on the enzyme(s), before the filling is enrobed with chocolate. The very viscous (and thus formable) filling then becomes less viscous with time, giving the creamy consistency desired. This results from the sub-optimal enzyme(s) conditions purposely created by withholding activation factors, which allows only a fraction of the enzyme(s) to be active, or allows all enzyme(s) to proceed at only a fraction of the biological rate [biologically, it's realistically a combination of both: a reduced number of functional enzymes, with the ones that do function having reduced catalytic kinetics/rates].
- Cigarettes use inverted sugar as a casing to add flavour.[14]
- Alcoholic beverage manufacturers often add invert sugar in the production of drinks like gin, beer and sparkling wines for flavouring. Candi sugar, similar to invert sugar, is used in the brewing of Belgian-style beers to boost alcohol content without drastically increasing the body of the beer; it is frequently found in the styles of beer known as dubbel and tripel.[8]
- Cadbury Creme Eggs are filled with inverted sugar syrup produced by processing fondant with invertase.[15][16]
- Sour Patch Kids also contain inverted sugar to add sweet flavor.
See also
Notes
- ↑ This formula presentation is based on sucrose or sugar mass, and is not to be confused with a true-percentage-based formula. It is analogous to baker's percentages. The acid per-cent is low and presumes no neutralization, higher amounts are commonly used. The water per-cent may be changed for a variety of reasons, including the inversion time as well as boiling time it takes to reach the final temperature.
- ↑ This formula is based on cream of tartar or potassium bitatrate mass. While this method does not measure the syrup's pH, it will neutralize all the added cream of tartar. However, some of the acid may already have been neutralized by hard water.
References
- ↑ "What are the types of sugar?". The Sugar Association. Archived from the original on March 1, 2009.
- ↑ "Making simple syrup is an exercise in chemical reactions". A Word from Carol Kroskey. Archived from the original on July 14, 2007. Retrieved May 1, 2006.
In addition to increased moisture retention ability, converting sucrose to invert syrup has two other interesting results: increased sweetness and better solubility. On a sweetness scale where sucrose is set at 100, invert syrup ranks about 130.
- ↑ "Types of Sugar". The Sugar Association. Retrieved October 14, 2014.
Because fructose is sweeter than either glucose or sucrose, invert sugar is sweeter than white sugar.
- ↑ Schiweck, Hubert; Clarke, Margaret; Pollack, Günter (2007). "Sugar". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_345.pub2.
- ↑ Van Damme, Eddy. "Invert sugar recipe". Retrieved September 27, 2012.
- 1 2 W. Minifie, Bernard (1989). Chocolate, Cocoa and Confectionery: Science and Technology (3rd ed.). Aspen Publishers, Inc. p. 246. ISBN 083421301X. Retrieved July 3, 2014 – via Google Books.
- ↑ Ranken, Michael D.; Kill, R.C.; Baker, C., eds. (1997). Food Industries Manual (24th ed.). London: Blackie Academic & Professional. pp. 407–408. ISBN 0751404047. Retrieved June 30, 2014 – via Google Books.
Commercially, invert sugar is prepared as a syrup of about 70% soluble solids concentration. Invert sugar can be produced by holding a 65% sucrose solution containing 0.25% hydrochloric acid at 50°C (122°F) for one hour. Sodium bicarbonate should then be added to neutralize the acid.
- 1 2 3 "The Sugar Beet". Vol. 25 no. 10. Philadelphia: H.C. Baird & Company. 1904. pp. 171–172. Retrieved July 4, 2014 – via Google Books.
- ↑ Lean, Michael E.J. (2006). Fox and Cameron's Food Science, Nutrition & Health (7th ed.). Boca Raton, FL: CRC Press. p. 110. ISBN 9780340809488. Retrieved July 1, 2014 – via Google Books.
- ↑ Morrison, Abraham Cressy (1904). The Baking Powder Controversy. vol. 1. New York: The American Baking Powder Association. p. 154. Retrieved July 2, 2014 – via Google Books.
The best cream of tarter baking powder on the market contains about 28 per cent of bicarbonate of soda. To neutralize this quantity ... 62.6 per cent of cream of tartar is required. This quantity will leave in the food 70 per cent of anhydrous Rochelle Salts.
- ↑ Maga, Joseph A.; Tu, Anthony T., eds. (1995). Food Additive Toxicology. New York: Marcel Dekker. p. 71, table 24. ISBN 0824792459. Retrieved July 3, 2014 – via Google Books.
- 1 2 Pennington, Neil L.; Baker, Charles W., eds. (1990). Sugar: User's Guide To Sucrose. New York: Van Nostrand Reinhold. pp. 108–109. ISBN 0442002971. Retrieved July 1, 2014 – via Google Books.
- ↑ Potter, Norman N.; Hotchkiss, Joseph H., eds. (1998). Food Science (5th ed.). Aspen Publishers. p. 468, table 20.3. ISBN 083421265X. Retrieved July 1, 2014 – via Google Books.
- ↑ "BAT Global Ingredients". British American Tobacco. Retrieved August 27, 2009.
- ↑ "Creme Egg". Cadbury. Retrieved April 10, 2015.
- ↑ LaBau, Elizabeth. "What is Invertase?". About.com. Retrieved April 10, 2015.
External links

- "Invertase". Retrieved November 27, 2012.