Trehalose
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3S,4S,5R,6R)-2-(Hydroxymethyl)-6-[(2R,3R,4S,5S,6R)-3,4,
5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxane-3,4,5-triol | |
| Other names
α,α‐Trehalose; α-D-glucopyranosyl-(1→1)-α-D-glucopyranoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.490 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C12H22O11 (anhydride) | |
| Molar mass | 342.296 g/mol (anhydrous) 378.33 g/mol (dihydrate) |
| Appearance | White orthorhombic crystals |
| Density | 1.58 g/cm3 at 24 °C |
| Melting point | 203 °C (397 °F; 476 K) (anhydrous) 97 °C (dihydrate) |
| 68.9 g per 100 g at 20 °C[1] | |
| Solubility | soluble in ethanol, insoluble in diethyl ether and benzene[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trehalose is a sugar consisting of two molecules of glucose. It is also known as mycose or tremalose. Some bacteria, fungi, plants and invertebrate animals synthesize it as a source of energy, and to survive freezing and lack of water.
Extracting trehalose was once a difficult and costly process, but around 2000, the Hayashibara company (Okayama, Japan) discovered an inexpensive extraction technology from starch.[3][4] Trehalose has high water retention capabilities, and is used in food, cosmetics and as a drug.
Structure
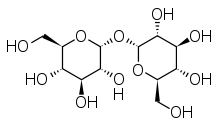
Trehalose is a disaccharide formed by a 1,1-glycosidic bond between two α-glucose units. Two other isomers are not found in nature.[5] It is found in nature as a disaccharide and also as a monomer in some polymers.[5]
Synthesis
At least three biological pathways support trehalose biosynthesis.[5] An industrial process can derive trehalose from corn starch.[6]
Properties
Chemical
Trehalose is a nonreducing sugar formed from two glucose units joined by a 1–1 alpha bond, giving it the name α-D-glucopyranosyl-(1→1)-α-D-glucopyranoside. The bonding makes trehalose very resistant to acid hydrolysis, and therefore is stable in solution at high temperatures, even under acidic conditions. The bonding keeps nonreducing sugars in closed-ring form, such that the aldehyde or ketone end groups do not bind to the lysine or arginine residues of proteins (a process called glycation). Trehalose is less soluble than sucrose, except at high temperatures (>80 °C). Trehalose forms a rhomboid crystal as the dihydrate, and has 90% of the calorific content of sucrose in that form. Anhydrous forms of trehalose readily regain moisture to form the dihydrate. Anhydrous forms of trehalose can show interesting physical properties when heat-treated.
Trehalose aqueous solutions show a concentration-dependent clustering tendency. Owing to their ability to form hydrogen bonds, they self-associate in water to form clusters of various sizes. All-atom molecular dynamics simulations showed that concentration of 1.5–2.2 molar, allow trehalose molecular clusters to percolate and form large, continuous aggregates.[7]
Trehalose directly interacts with nucleic acids, facilitates melting of double stranded DNA and stabilizes single-stranded nucleic acids.[8]
Biological
Organisms ranging from bacteria, yeast, fungi, insects, invertebrates, and lower and higher plants have enzymes that can make trehalose.[5] In nature, trehalose can be found in plants, and microorganisms. In animals, trehalose is prevalent in shrimp, and also in insects, including grasshoppers, locusts, butterflies, and bees, in which trehalose serves as blood-sugar. Trehalose is then broken down into glucose by the catabolic enzyme trehalase for use. Trehalose is also present in the nutrition exchange liquid of hornets and their larvae.
Trehalose is the major carbohydrate energy storage molecule used by insects for flight. One possible reason for this is that the glycosidic linkage of trehalose, when acted upon by an insect trehalase, releases two molecules of glucose, which is required for the rapid energy requirements of flight. This is double the efficiency of glucose release from the storage polymer starch, for which cleavage of one glycosidic linkage releases only one glucose molecule.
In plants, trehalose is seen in sunflower seeds, moonwort, Selaginella plants,[9] and sea algae. Within the fungi, it is prevalent in some mushrooms, such as shiitake (Lentinula edodes), oyster, king oyster, and golden needle.[10]
Even within the plant kingdom, Selaginella (sometimes called the resurrection plant), which grows in desert and mountainous areas, may be cracked and dried out, but will turn green again and revive after rain because of the function of trehalose.[9]
The two prevalent theories as to how trehalose works within the organism in the state of cryptobiosis are the vitrification theory, a state that prevents ice formation, or the water displacement theory, whereby water is replaced by trehalose.[11]
Nutritional and dietary properties
Trehalose is rapidly broken down into glucose by the enzyme trehalase, which is present in the brush border of the intestinal mucosa of omnivores (including humans) and herbivores.[12]:135 It causes less of a spike in blood sugar than glucose.[13] Trehalose has about 45% the sweetness of sucrose at concentrations above 22%, but when the concentration is reduced, its sweetness decreases more quickly than that of sucrose, so that a 2.3% solution tastes 6.5 times less sweet as the equivalent sugar solution.[14]:444
It is commonly used in prepared frozen foods, like ice cream, because it lowers the freezing point of foods.[13]
The Cargill corporation promotes the use of its brand of trehalose, "Treha," as a substance that "enhances and intensifies certain flavors to bring out the best in your products."[15]
Deficiency of trehalase enzyme is unusual in humans, except in the Greenlandic Inuit, where it occurs in 10–15% of the population.[16]:197
Medical use
Trehalose is an ingredient, along with hyaluronic acid, in an artificial tears product used to treat dry eye.[17][18]
Research
In 1832, H.A.L. Wiggers discovered trehalose in an ergot of rye,[19] and in 1859 Marcellin Berthelot isolated it from Trehala manna, a substance made by weevils and named it trehalose.[20]
In January 2018 research was published showing that some strains of Clostridium difficile found in the human gut microbiome are especially responsive to trehalose; the study found that in the lab and in mice, these strains grew better and produced more toxins when fed trehalose. The authors pointed out a correlation between the timing of the introduction of trehalose into the food supply when the cost of production came down around 2000, and a rise in cases of hospital-acquired C. difficile infections, and suggested that trehalose might be causing the rise in infections. It was not known at that time whether trehalose actually caused the rise in C. difficile infections or affects C. difficile in the human gut.[13][21][22]
See also
References
- ↑ Higashiyama, Takanobu (2002). "Novel functions and applications of trehalose" (PDF). Pure Appl. Chem. 74 (7): 1263–1269. doi:10.1351/pac200274071263.
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 3–534. ISBN 0-8493-0594-2.
- ↑ Cargill, Incorporated (30 May 2011). "Cargill, Hayashibara to Introduce Trehalose Sweetener to the Americas". PR Newswire. Retrieved 2011-07-31.
- ↑ "JAPAN: Cargill, Hayashibara to Introduce Trehalose Sweetener to the Americas". just-food.com. Retrieved 2 February 2013.
- 1 2 3 4 Elbein, Alan; Y T Pan, Irina Pastuszak and David Carroll (2003). "New insights on trehalose: a multifunctional molecule". Glycobiology. 13 (4): 17R–27R. doi:10.1093/glycob/cwg047. PMID 12626396. Retrieved 21 October 2013.
- ↑ "GRAS Notification for Hayashibara Trehalose" (PDF). Food and Drug Administration. May 3, 2000: 10.
- ↑ Sapir, Liel; Harries, Daniel (2011). "Linking Trehalose Self-Association with Binary Aqueous Solution Equation of State". J. Phys. Chem. B. 115 (4): 624–634. doi:10.1021/jp109780n. PMID 21186829.
- ↑ Bezrukavnikov, Sergey; Mashaghi, Alireza; Wijk, Roeland J. van; Gu, Chan; Yang, Li Jiang; Gao, Yi Qin; Tans, Sander J. (27 August 2014). "Trehalose facilitates DNA melting: a single-molecule optical tweezers study". Soft Matter. 10 (37): 7269. Bibcode:2014SMat...10.7269B. doi:10.1039/C4SM01532K. PMID 25096217. Retrieved 5 January 2018.
- 1 2 Zentella, Rodolfo; Mascorro-Gallardo, José O.; Van Dijck, Patrick; Folch-Mallol, Jorge; Bonini, Beatriz; Van Vaeck, Christophe; Gaxiola, Roberto; Covarrubias, Alejandra A.; Nieto-Sotelo, Jorge; Thevelein, Johan M.; Iturriaga, Gabriel (1999). "A Selaginella lepidophylla Trehalose-6-Phosphate Synthase Complements Growth and Stress-Tolerance Defects in a Yeasttps1 Mutant". Plant Physiology. 119 (4): 1473–1482. doi:10.1104/pp.119.4.1473. PMC 32033. PMID 10198107.
- ↑ Reis, FS; Barros, L; Martins, A; Ferreira, IC (February 2012). "Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study" (PDF). Food and Chemical Toxicology. 50 (2): 191–7. doi:10.1016/j.fct.2011.10.056. PMID 22056333.
- ↑ Sola-Penna M, Meyer-Fernandes JR; Meyer-Fernandes (1998). "Stabilization against thermal inactivation promoted by sugars on enzyme structure and function: why is trehalose more effective than other sugars?". Archives of Biochemistry and Biophysics. 360 (1): 10–14. doi:10.1006/abbi.1998.0906. PMID 9826423.
- ↑ Mathlouthi, M., ed. (1999). Food packaging and preservation. Gaithersburg, Md.: Aspen Publishers. ISBN 978-0-8342-1349-4. Retrieved 25 June 2014.
- 1 2 3 Collins, Francis (9 January 2018). "Has an Alternative to Table Sugar Contributed to the C. Diff. Epidemic?". NIH Director's Blog.
- ↑ O'Brien-Nabors, Lyn, ed. (2012). Alternative sweeteners (4th ed.). Boca Raton: CRC Press. ISBN 978-1-4398-4614-8. Retrieved 25 June 2014.
- ↑ "Trehalose supplier". Retrieved 14 June 2018.
- ↑ Kohlmeier, Martin (2003). Nutrient Metabolism. Burlington: Elsevier. ISBN 978-0-08-053789-4. Retrieved 25 June 2014.
- ↑ Pinto-Bonilla JC, Del Olmo-Jimeno A, Llovet-Osuna F, Hernandez-Galilea E (2015). "A randomized cross over study comparing trehalose/hyaluronate eyedrops and standard treatment: patient satisfaction in the treatment of dry dye syndrome". Ther Clin Risk Manag. 11: 595–603. doi:10.2147/TCRM.S77091. PMC 4403513. PMID 25926736.
- ↑ Pucker AD, Ng SM, Nichols JJ (2016). "Over the counter (OTC) artificial tear drops for dry eye syndrome". Cochrane Database Syst Rev. 2: CD009729. doi:10.1002/14651858.CD009729.pub2. PMC 5045033. PMID 26905373.
- ↑ Wiggers, H. A. L. (1832). "Untersuchung über das Mutterkorn, Secale cornutum". Annalen der Pharmacie. 1 (2): 129–182. doi:10.1002/jlac.18320010202.
- ↑ Tillequin, F (2009). "Trehala, a meeting point between zoology, botany, chemistry, and biochemistry". Revue d'histoire de la pharmacie. 57 (362): 163–72. PMID 20027793.
- ↑ Ballard, Jimmy D. (18 January 2018). "Pathogens boosted by food additive". Nature. pp. 285–286. Bibcode:2018Natur.553..285B. doi:10.1038/d41586-017-08775-4. PMID 29345660.

- ↑ Collins, J; Robinson, C; Danhof, H; Knetsch, CW; van Leeuwen, HC; Lawley, TD; Auchtung, JM; Britton, RA (18 January 2018). "Dietary trehalose enhances virulence of epidemic Clostridium difficile". Nature. 553 (7688): 291–294. Bibcode:2018Natur.553..291C. doi:10.1038/nature25178. PMC 5984069. PMID 29310122.
Further reading
- Ballard, Jimmy D. (21 December 2017). "News and Views: Pathogens boosted by food additive". Nature. , discussing 2010 paper: Collins, J; Robinson, C; Danhof, H; Knetsch, CW; van Leeuwen, HC; Lawley, TD; Auchtung, JM; Britton, RA (3 January 2018). "Dietary trehalose enhances virulence of epidemic Clostridium difficile". Nature. Bibcode:2018Natur.553..291C. doi:10.1038/nature25178. PMID 29310122. .
External links
