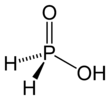
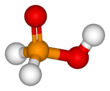
Hypophosphorous acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphinic acid | |||
| Other names
Hydroxy(oxo)-λ5-phosphane Hydroxy-λ5-phosphanone | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.026.001 | ||
| KEGG | |||
PubChem CID |
| ||
| UN number | UN 3264 | ||
| |||
| |||
| Properties | |||
| H3PO2 | |||
| Molar mass | 66.00 g/mol | ||
| Appearance | colorless, deliquescent crystals or oily liquid | ||
| Density | 1.493 g/cm3[2]
1.22 g/cm3 (50 wt% aq. solution) | ||
| Melting point | 26.5 °C (79.7 °F; 299.6 K) | ||
| Boiling point | 130 °C (266 °F; 403 K) decomposes | ||
| miscible | |||
| Solubility | very soluble in alcohol, ether | ||
| Acidity (pKa) | 1.2 | ||
| Conjugate base | Phosphinate | ||
| Structure | |||
| pseudo-tetrahedral | |||
| Hazards | |||
| Safety data sheet | JT Baker | ||
| Flash point | Non-flammable | ||
| Related compounds | |||
Related phosphorus oxoacids |
Phosphorous acid Phosphoric acid | ||
Related compounds |
Sodium hypophosphite Barium hypophosphite | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hypophosphorous acid is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. Inorganic chemists refer to the free acid by this name (also as "HPA"), or the acceptable name of phosphinic acid. It is a colorless high-melting compound, which is soluble in water, dioxane, and alcohols. The formula for blue acid is generally written H3PO2, but a more descriptive presentation is HOP(O)H2 which highlights its monoprotic character. Salts derived from this acid are called phosphinates (hypophosphites).
HOP(O)H2 exists in equilibrium with the minor tautomer HP(OH)2. Sometimes the minor tautomer is called hypophosphorous acid and the major tautomer is called phosphinic acid.
Preparation and availability
Hypophosphorous acid was first prepared in 1816 by the French chemist Pierre Louis Dulong (1785–1838).[3]
The acid is prepared industrially via a two step process: Firstly, hypophosphite salts of the alkali and alkaline earth metals result from the reaction of white phosphorus with hot aqueous solution of the appropriate hydroxide, e.g. Ca(OH)2.
- P4 + 4 OH− + 4 H2O → 4 H
2PO−
2 + 2 H2
This is then protonated by a strong, non-oxidizing acid to give the free hypophosphorous acid .
- H
2PO−
2 + H+ → H3PO2
HPA is usually supplied as a 50% aqueous solution. Anhydrous acid cannot be obtained by simple evaporation of the water, as the acid ready oxidises to phosphorous acid and phosphoric acid and also disproportionates to phosphorous acid and phosphine. Pure anhydrous hypophosphorous acid can be formed by the continuous extraction of aqueous solutions with diethyl ether.[4]
Uses
Its main industrial use is for electroless nickel plating (Ni–P), although it is primarily used as a salt (sodium hypophosphite).[5] In organic chemistry, H3PO2 can be used for the reduction of arenediazonium salts, converting ArN+
2 to Ar–H.[6][7][8] When diazotized in a concentrated solution of hypophosphorous acid, an amine substituent can be removed from arenes, selectively over alkyl amines. Owing to its ability to act as a mild reducing agent and oxygen scavenger it is sometimes used as an additive in Fischer esterification reactions, where it prevents the formation of colored impurities. Hypophosphorous acid is also used in the formulation of pharmaceuticals, discoloration of polymers, water treatment, retrieval of precious or non-ferrous metals.
DEA List I chemical status
Because hypophosphorous acid can reduce elemental iodine to form hydroiodic acid, which is a reagent effective for reducing ephedrine or pseudoephedrine to methamphetamine,[9] the United States Drug Enforcement Administration designated hypophosphorous acid (and its salts) as a List I precursor chemical effective November 16, 2001.[10] Accordingly, handlers of hypophosphorous acid or its salts in the United States are subject to stringent regulatory controls including registration, recordkeeping, reporting, and import/export requirements pursuant to the Controlled Substances Act and 21 CFR §§ 1309 and 1310.[10][11][12]
Organophosphinic acids (Phosphinates)
Organophosphinic acids have the formula R2PO2H. The two hydrogen atoms directly bound to phosphorus in phosphinic acid are replaced by organic groups. For example, formaldehyde and H3PO2 react to give (HOCH2)2PO2H. Similarly, phosphinic acid adds to Michael acceptors, for example with acrylamide it gives H(HO)P(O)CH2CH2C(O)NH2. The Cyanex family of dialkylphosphinic acids are used in hydrometallurgy to extract metals from ores.
Inorganic derivatives
Few metal complexes have been prepared from H3PO2, one example is Ni(O2PH2)2.
Sources
- Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ChemicalLand21 Listing
- Corbridge, D. E. C. Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier. ISBN 0-444-89307-5.
- Popik, V. V.; Wright, A. G.; Khan, T. A.; Murphy, J. A. (2004). "Hypophosphorous Acid". In Paquette, L. Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289.
- Rich, D. W.; Smith, M. C. (1971). Electroless Deposition of Nickel, Cobalt & Iron. Poughkeepsie, NY: IBM Corporation.
References
- ↑ Petrucci,, Ralph H. (2007). General Chemistry (9th ed.). p. 946.
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑ Dulong first prepared hypophosphorous acid (acide hypo-phosphoreux) by adding barium phosphide (Ba3P2) to water, which yielded phosphine gas (PH3), barium phosphate, and barium hypophosphite. Since the phosphine gas left the solution and the barium phosphate precipitated, only the barium hypophosphite remained in solution. Hypophosphorous acid could then be obtained from the filtrate by adding sulfuric acid, which precipitated barium sulfate, leaving hypophosphorous acid in solution. See:
- Dulong (1816) "Extrait d'un mémoire sur les combinaisons du phosphore avec l'oxigène" (Extract from a memoir on the compounds of phosphorus with oxygen), Annales de Chimie et de Physique, 2 : 141–150. [in French]
- Graham, Thomas, Elements of Inorganic Chemistry, 2nd ed. (Philadelphia, Pennsylvania: Blanchard and Lea, 1858), p. 316.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 513. ISBN 0-08-037941-9.
- ↑ Abrantes, L. M. (1994). "On the Mechanism of Electroless Ni–P Plating". Journal of The Electrochemical Society. 141 (9): 2356. doi:10.1149/1.2055125.
- ↑ William H. Brown; Brent L. Iverson; Eric Anslyn; Christopher S. Foote (2013). Organic Chemistry. Cengage Learning. p. 1003. ISBN 9781133952848.
- ↑ Robison, M. M.; Robison, B. L. "2,4,6-Tribromobenzoic acid". Organic Syntheses. 36: 94. ; Collective Volume, 4
- ↑ Kornblum, N. "3,3′-Dimethoxybiphenyl and 3,3′-dimethylbiphenyl". Organic Syntheses. 21: 30. ; Collective Volume, 3
- ↑ Gordon, P. E.; Fry, A. J.; Hicks, L. D. (23 August 2005). "Further studies on the reduction of benzylic alcohols by hypophosphorous acid/iodine" (PDF). ARKIVOC. 2005 (vi): 393–400. ISSN 1424-6376.
- 1 2 66 FR 52670—52675. 17 October 2001.
- ↑ 21 CFR 1309
- ↑ 21 USC, Chapter 13 (Controlled Substances Act)