Phosphinate

Phosphinates or hypophosphites are a class of phosphorus compounds conceptually based on the structure of hypophosphorous acid. IUPAC prefers the term phosphinate in all cases, however in practice hypophosphite is usually used to describe inorganic species (e.g. sodium hypophosphite), while phosphinate typically refers to organophosphorus species.
Hypophosphites

The hypophosphite ion is H
2PO−
2. The salts are prepared by heating white phosphorus in warm aqueous alkali e.g. Ca(OH)2:[1]
- P4 + 2 Ca(OH)2 + 4 H2O → 2 Ca(H2PO2)2 + 2 H2
Hypophosphites are reducing agents:[1]
- H
2PO−
2 + 3OH− → HPO2−
3 + 2H2O + 2e−
Hypophosphites are used in electroless nickel plating as the reducing agent to deposit for example Ni metal from Ni salts.[1] The hypophosphite ion is thermodynamically unstable, and disproportionates on heating to phosphine gas and phosphate salts:
Organophosphinates
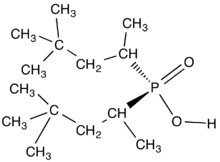
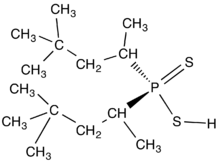
Organophosphinates are organophosphorus compounds with the formula OP(OR)R2. They can be synthetized by the Michaelis–Arbuzov reaction. Phosphinites P(OR)R2 can be oxidized into phosphinates.
Dialkylphosphinic acids are organophosphorus compounds with the formula R2PO2H, where R is any alkyl group. They are phosphorus(V) compounds with tetrahedral molecular geometry. Under the brand names Aerophine and Cyanex, they are used in extraction and separation, i.e., hydrometallurgy, of metal salts from ore extracts.[2] Characteristically the organic substituents are branched to confer solubility and preclude crystallization.[3]
The dithiodialkyphosphinic acids (R2PS2H) are related to the diorganodithiophosphates with the formula (RO)2PS2H, which are also used as complexing agents in the purification of metals. The phosphates are more prone to hydrolysis owing to the greater lability of the RO-P linkage vs the direct C-P bond.
See also
- Phosphine - PR3
- Phosphine oxide - OPR3
- Phosphite - P(OR)3
- Phosphonate - OP(OR)2R
- Phosphate - OP(OR)3
References
- 1 2 3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 512. ISBN 0-08-037941-9.
- ↑ J. Svara, N. Weferling, T. Hofmann "Phosphorus Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006. doi:10.1002/14356007.a19_545.pub2
- ↑ I.Yu. Fleitlikh, N.A. Grigorieva, V.I. Kuz'min, G.L. Pashkov "Redox processes during cobalt extraction with bis(2,4,4-trimethylpentyl)dithiophosphinic acid" Hydrometallurgy 2012, volumes 129–130, pp. 43–49. doi:10.1016/j.hydromet.2012.08.009