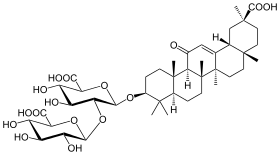
Glycyrrhizin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Epigen, Glycyron |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, intravenous |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic and by intestinal bacteria |
| Elimination half-life | 6.2-10.2 hours[1] |
| Excretion | Faeces, urine (0.31-0.67%)[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| E number |
E958 (glazing agents, ...) |
| ECHA InfoCard |
100.014.350 |
| Chemical and physical data | |
| Formula | C42H62O16 |
| Molar mass | 822.93 g/mol |
| 3D model (JSmol) | |
| Solubility in water | 1-10 mg/mL (20 °C) |
| |
| |
Glycyrrhizin (or glycyrrhizic acid or glycyrrhizinic acid) is the chief sweet-tasting constituent of Glycyrrhiza glabra (liquorice) root. Structurally, it is a saponin used as an emulsifier and gel-forming agent in foodstuffs and cosmetics. Its aglycone is enoxolone assessed as a prodrug used in Japan to reduce the risk of liver cancer in people with chronic hepatitis C.[3][4]
Adverse effects
The most widely reported side effect of glycyrrhizin use via consumption of black licorice is reduction of blood potassium levels, which can affect body fluid balance and function of nerves.[5][6] Chronic consumption of black licorice, even in moderate amounts, is associated with an increase in blood pressure,[6] may cause irregular heart rhythm, and adverse interactions with prescription medicines.[5]
The effects on body fluids are related to the inhibition of cortisol metabolism within the kidney, subsequent stimulation of the mineralocorticoid receptors,[7] and decrease in blood levels of renin, potassium, and aldosterone, which collectively lead to increases in blood pressure.[6]
Depending on amount and frequency of ingesting black licorice, other side effects may include:[5][8]
- Edema
- Lethargy
- Headache
- Paralysis
- Transient visual loss
- Torsades de pointes
- Tachycardia
- Cardiac arrest
- Reduced testosterone
- Premature birth
- Acute kidney failure
- Muscle weakness
- Myopathy
- Myoglobinuria
- Rhabdomyolysis
- Increased body weight
Research
Glycyrrhizin is under laboratory and preliminary clinical research for its possible activity against common viruses, such as hepatitis C.[4] In vitro, glycyrrhizin inhibits the enzyme 11β-hydroxysteroid dehydrogenase.[8]
Pharmacokinetics
After oral ingestion, glycyrrhizin is first hydrolysed to 18β-glycyrrhetinic acid by intestinal bacteria. After complete absorption from the gut, β-glycyrrhetinic acid is metabolised to 3β-monoglucuronyl-18β-glycyrrhetinic acid in the liver. This metabolite then circulates in the bloodstream. Consequently, its oral bioavailability is poor. The main part is eliminated by bile and only a minor part (0.31–0.67%) by urine.[9] After oral ingestion of 600 mg of glycyrrhizin the metabolite appeared in urine after 1.5 to 14 hours. Maximal concentrations (0.49 to 2.69 mg/l) were achieved after 1.5 to 39 hours and metabolite can be detected in the urine after 2 to 4 days.[9]
Flavouring properties
Glycyrrhizin is obtained as an extract from licorice root after maceration and boiling in water.[10] Licorice extract (glycyrrhizin) is sold in the United States as a liquid, paste, or spray-dried powder.[10] When in specified amounts, it is approved for use as a flavor and aroma in manufactured foods, beverages, candies, dietary supplements, and seasonings.[10] It is 30 to 50 times as sweet as sucrose (table sugar).[8][11]
See also
References
- ↑ van Rossum, TG; Vulto, AG; Hop, WC; Schalm, SW (December 1999). "Pharmacokinetics of intravenous glycyrrhizin after single and multiple doses in patients with chronic hepatitis C infection". Clinical Therapeutics. 21 (12): 2080–90. doi:10.1016/S0149-2918(00)87239-2. PMID 10645755.
- ↑ Ploeger, B; Mensinga, T; Sips, A; Seinen, W; Meulenbelt, J; DeJongh, J (May 2001). "The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling". Drug Metabolism Reviews. 33 (2): 125–47. doi:10.1081/DMR-100104400. PMID 11495500.
- ↑ Arase, Yasuji; Ikeda, Kenji; Murashima, Naoya; Chayama, Kazuaki; Tsubota, Akihito; Koida, Isao; Suzuki, Yoshiyuki; Saitoh, Satoshi; Kobayashi, Masahiro; Kumada, Hiromitsu (15 April 1997). "The long term efficacy of glycyrrhizin in chronic hepatitis C patients". Cancer. 79 (8): 1494–1500. doi:10.1002/(SICI)1097-0142(19970415)79:8<1494::AID-CNCR8>3.0.CO;2-B.
- 1 2 Fiore, C; Eisenhut, M; Krausse, R; Ragazzi, E; Pellati, D; Armanini, D; Bielenberg, J (2008). "Antiviral effects of Glycyrrhiza species". Phytotherapy Research. 22 (2): 141–8. doi:10.1002/ptr.2295. PMID 17886224.
- 1 2 3 "Black Licorice: Trick or Treat?". US Food and Drug Administration. 30 October 2017. Retrieved 15 December 2017.
- 1 2 3 Penninkilampi, R; Eslick, E. M; Eslick, G. D (2017). "The association between consistent licorice ingestion, hypertension and hypokalaemia: A systematic review and meta-analysis". Journal of Human Hypertension. 31 (11): 699–707. doi:10.1038/jhh.2017.45. PMID 28660884.
- ↑ Ferrari, P.; Sansonnens, A.; Dick, B.; Frey, F. J. (2001). "In Vivo 11 -HSD-2 Activity: Variability, Salt-Sensitivity, and Effect of Licorice". Hypertension. 38 (6): 1330–6. doi:10.1161/hy1101.096112. PMID 11751713.
- 1 2 3 Asl, MN; Hosseinzadeh, H (1 June 2008). "Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds". Phytotherapy Research. 22 (6): 709–24. doi:10.1002/ptr.2362. PMID 18446848.
- 1 2 Kočevar Glavač, Nina; Kreft, Samo (2012). "Excretion profile of glycyrrhizin metabolite in human urine". Food Chemistry. 131: 305–308. doi:10.1016/j.foodchem.2011.08.081.
- 1 2 3 "Sec. 184.1408 Licorice and licorice derivatives". US Food and Drug Administration, Code of Federal Regulations Title 21, 21CFR184.1408. 1 April 2017. Retrieved 15 December 2017.
- ↑ "Glycyrrhizic Acid". PubChem. National Institutes of Health. Retrieved 24 February 2014.
External links
