Econazole
 | |
| Clinical data | |
|---|---|
| Trade names | Spectazole, Ecostatin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684049 |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.043.932 |
| Chemical and physical data | |
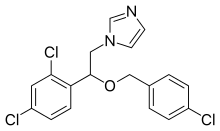
| Formula | C18H15Cl3N2O |
| Molar mass | 381.683 g/mol |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Econazole (commonly used as the nitrate salt) is an antifungal medication of the imidazole class.[1] It is sold under the brand names Spectrazole (United States) and Ecostatin (Canada), among others. It is a component of Pevisone and Ecoderm-TA[2] (econazole/triamcinolone).
Medical uses
Econazole is used as a cream to treat skin infections such as athlete's foot, tinea, pityriasis versicolor, ringworm, and jock itch. It is also sold in Canada under the brand name Ecostatin as vaginal ovules to treat vaginal thrush.
Econazole nitrate exhibits strong anti-feeding properties against the keratin-digesting common clothes moth Tineola bisselliella.[3]
Adverse effects
About 3% of patients treated with econazole nitrate cream reported side effects. The most common symptoms were burning, itching, erythema, and one outbreak of a pruritic rash.[4]
Synthesis
Imidazoles devoid of the nitro group no longer have any antiprotozoal activity, however, such drugs are effective antifungal agents.
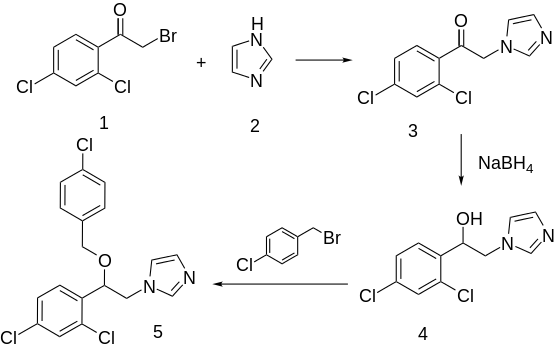
Alkylation of imidazole (2) with bromoketone (1) prepared from o,p-dichloroacetophenone affords the displacement product (3). Reduction of the ketone with sodium borohydride gives the corresponding alcohol (4). Alkylation of the alkoxide from that alcohol with p-chlorobenzyl chloride leads to econazole (5); alkylation with o,p-dichlorobenzyl chloride gives miconazole.
References
- ↑ Thienpont, D; Van Cutsem, J; Van Nueten, JM; Niemegeers, CJ; Marsboom, R (1975). "Bilogical and toxicological properties of econazole, a broad-spectrum antimycotic". Arzneimittel-Forschung. 25 (2): 224–30. PMID 1173036.
- ↑ Product descriptions at REPHCO Pharmaceuticals Limited, Bangladesh. Retrieved June 2012
- ↑ Sunderland, M. R.; Cruickshank, R. H.; Leighs, S. J. (2014). “The efficacy of antifungal azole and antiprotozoal compounds in protection of wool from keratin-digesting insect larvae”. Textile Research Journal 84 (9): 924–931. http://trj.sagepub.com/content/84/9/924
- ↑ Perrigo Econazole Nitrate Cream Product Description. 2012.
- ↑ Godefroi, E. F.; Heeres, J.; Van Cutsem, J.; Janssen, P. A. J. (1969). "Preparation and antimycotic properties of derivatives of 1-phenethylimidazole". Journal of Medicinal Chemistry. 12 (5): 784. doi:10.1021/jm00305a014.