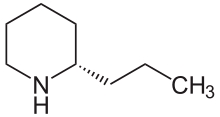
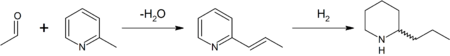Coniine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2-propylpiperidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.621 |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C8H17N | |
| Molar mass | 127.23 g·mol−1 |
| Melting point | −2 °C (28 °F; 271 K) |
| Boiling point | 166 to 167 °C (331 to 333 °F; 439 to 440 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Coniine refers to a poisonous chemical compound, an alkaloid present in and isolable from poison hemlock (Conium maculatum), where its presence has been a source of significant economic, medical, and historico-cultural interest; coniine is also produced by the yellow pitcher plant (Sarracenia flava), and fool's parsley (Aethusa cynapium). Its ingestion and extended exposure are toxic to humans and all classes of livestock; its mechanism of poisoning involves disruption of the central nervous system, with death caused by respiratory paralysis. The biosynthesis of coniine contains as its penultimate step the non-enzymatic cyclisation of 5-oxooctylamine to γ-coniceine, a Schiff base differing from coniiine only by its carbon-nitrogen double bond in the ring. This pathway results in natural coniine that is a mixture—a racemate—composed of two enantiomers, the stereoisomers (S)-(+)-coniine, and (R)-(−)-coniine, depending on the direction taken by the chain that branches from the ring. Both enantiomers are toxic, with the (R)-(−) enantiomer being the more biologically active and toxic of the two in general. Coniine holds a place in organic chemistry history as being the first of the important class of alkaloids to be synthesized, by Albert Ladenburg in 1886, and it has been synthesized by humans in a number of unique ways, through to modern times.
Hemlock poisoning has been a periodic human concern, a regular veterinary concern, and has had significant occurrences in human and cultural history. Notably, in 399 BC, Socrates, when he was sentenced to death, chose to die by drinking a coniine-containing mixture of poison hemlock.
Natural origins
Poison hemlock
Poison hemlock (Conium maculatum) contains highly toxic amounts of coniine, where it contributes to hemlock's fetid smell; in addition, it contains trace amounts of other similarly poisonous alkaloids. Ingesting less than a tenth of a gram of coniine can be fatal for adult humans; this is approximately six to eight hemlock leaves. The seeds and roots are also toxic, more so than the leaves. While hemlock toxicity primarily results from consumption, poisoning can also result from inhalation, and from skin contact.[1]

The presence of hemlock on farmland is an issue for livestock farmers because animals will eat it if they are not well fed or the hemlock is mixed in with pasture grass. Farmers also need to be careful that the hay fed to animals does not contain hemlock.[2] Poison hemlock is most poisonous in the spring when the concentration of γ-coniciene (the precursor to other toxins) is at its peak.[3][4]
Poison hemlock grows quite tall, reaching heights of up to twelve feet.[5] The stalk of hemlock is green with purple spots and completely lacks hair. A biennial plant, hemlock produces leaves at its base the first year but no flowers. In its second year it produces white flowers in umbrella shaped clusters.[6] Hemlock can be confused with the wild carrot plant; however, this plant has a hairy stem without purple markings, grows less than three feet tall, and does not have clustered flowers.[1] While the hemlock plant is native to Europe and the Mediterranean region,[7] it has spread to every other continent excluding Antarctica.[8]

Yellow pitcher plant
The coniine alkaloid is found in Sarracenia flava, the yellow pitcher plant.[9] The yellow pitcher plant is a carnivorous plant found exclusively in the southeastern United States. The plant uses a mixture of sugar and coniine to simultaneously attract and poison insects, which then fall into a digestive tube. The naming of the plant arises from the shape of the opening to these tubes, which resembles a pitcher, which can grow up to three feet tall. The pitcher has a leaf above it that functions to prevent rain from diluting the digestive fluids deep in the tubes.[10]
There are no reports online of human poisoning via the yellow pitcher plant, perhaps because only a small portion of the plant contains coniine, or because it does not contain enough to produce toxicity. It is also not as widespread as hemlock and therefore is less likely to be contacted by humans.
Fool's parsley
The coniine alkaloid is also found in Aethusa cynapium, commonly known as fool's parsley.[11]
History of natural isolates
The history of coniine is understandably tied to the poison hemlock plant, since the natural product was not synthesizable until the 1880s.[12] The most famous hemlock poisoning occurred in 399 BCE, when the philosopher Socrates is believed to have consumed a liquid infused with hemlock to carry out his death sentence, his having been convicted of impiety toward the gods, and the corruption of youth.[13][14][15] Hemlock juice was often used to execute criminals in ancient Greece.[16]
Hemlock has had a limited medical use throughout history. The Greeks used it not just as capital punishment, but also as an antispasmodic and treatment for arthritis. Books from the 10th century attest to medical use by the Anglo-Saxons.[17] In the Middle Ages it was believed that hemlock could be used to cure rabies; in later European times it came to be associated with flying ointments in witchcraft. Native Americans used hemlock extract as arrow poison.[8]
While the yellow pitcher plant and fool's parsley also contain coniine, there are no reports of traditional uses for these plants.
Pharmacology and toxicology
The (R)-(−) enantiomer of coniine is the more biologically active, at least in one system (TE-671 cells expressing human fetal nicotinic neuromuscular receptors), and in mouse bioassay, the same enantiomer and the racemic mixture are about two-fold more toxic than the (S)-(+) enantiomer (see below).[18]
Coniine, as racemate or as pure enantiomer, paralyzes muscles when one or the other molecule blocks the nicotinic receptor on the post-synaptic membrane of the neuromuscular junction. This results, systemically, in a flaccid paralysis, an action similar to that of curare. Symptoms of paralysis generally occur within a half-hour, although death may take several hours. The central nervous system is not affected the person remains conscious and aware until respiratory paralysis results in cessation of breathing. The flaccid, muscular paralysis is an ascending paralysis, lower limbs being first affected. The person may have a hypoxic convulsion just prior to death, disguised by the muscular paralysis such that the person may just weakly shudder. Cause of death is lack of oxygen to the brain and heart as a consequence of respiratory paralysis, so that a poisoned person may recover if artificial ventilation can be maintained until the toxin is removed from the victim's system.
The LD50 values (in mouse, i.v. administered) for the R-(−) and S-(+) enantiomers, and the racemate, are approximately 7 and 12, and 8 milligrams per kilogram, respectively.[18]
Chemical properties
(+/–)-Coniine was first isolated by Giesecke,[19] but the formula was suggested by Blyth[20] and definitely established by Hoffmann.[21][22]
D-(S)-Coniine has since been determined to be a colorless alkaline liquid, with a penetrating odour and a burning taste; has D0° 0.8626 and D19° 0.8438, refractive index n23°D 1.4505, and is dextrorotatory, [α]19°D +15.7°. (See comments about the specific rotation below, under "Enantiomers".) L-(R)-Coniine has [α]21°D 15° and in other respects resembles its D-isomer, but the salts have slightly different melting points; the platinichloride has mp. 160 °C (Löffler and Friedrich report 175 °C), the aurichloride mp. 59 °C.[23][24]
Solubility
Coniine is slightly soluble (1 in 90) in cold water, less so in hot water, so that a clear cold solution becomes turbid when warmed. On the other hand, the base dissolves about 25% of water at room temperature. It mixes with alcohol in all proportions, is readily soluble in ether and most organic solvents. Coniine dissolves in carbon disulfide, forming a complex thiocarbamate.[25][26]
Crystallization
Coniine solidifies into a soft crystalline mass at −2 °C. It slowly oxidizes in the air. The salts crystallize well and are soluble in water or alcohol. The hydrochloride, B•HCl, crystallizes from water in rhombs, mp. 220 °C, [α]20°D +10.1°; the hydrobromide, in needles, mp. 211 °C, and the D-acid tartrate, B•C4H6O6•2 H2O, in rhombic crystals, mp. 54 °C. The platinichloride, (B•HCl)2•PtCl4•H2O, separates from concentrated solution as an oil, which solidifies to a mass of orange-yellow crystals, mp. 175 °C (dry). The aurichloride, B•HAuCl4, crystallizes on standing, mp. 77 °C. The picrate forms small yellow needles, mp. 75 °C, from hot water. The 2,4-dinitrobenzoyl- and 3,5-dinitrobenzoyl-derivates have mps. 139.0–139.5 °C and 108–9 °C respectively.[27] The precipitate afforded by potassium cadmium iodide solution is crystalline, mp. 118 °C, while that given by nicotine with this reagent is amorphous.
Color changes
Coniine gives no coloration with sulfuric or nitric acid. Sodium nitroprusside gives a deep red color, which disappears on warming, but reappears on cooling, and is changed to blue or violet by aldehydes.[28]
Enantiomers
Naturally-occurring coniine is present in Conium maculatum as a mixture of the R-(−)- and S-(+)- enantiomers.[18] The stereochemical composition of "coniine" is a matter of some importance, since its two enantiomers do not have identical biological properties,[18] and many of the older pharmacological studies on this compound were carried out using the naturally-occurring isomeric mixture.
The common criterion for enantiomeric homogeneity is the specific rotation, [α]D, a value that depends on such factors as temperature, solvent and concentration of the analyte. Modern values for the specific rotation of the enantiomers of coniine are as follows:
- S-(+)-coniine, identical to D-(+)-coniine, has [α]D = +8.4° (c = 4.0, in CHCl3).[29] These authors note that Ladenburg's value,[30] +15°, is for a "neat" sample, undiluted with any solvent.
- A similarly high value of +16° for the [α]D of "coniine" is given, without explicit citation of the source, in The Merck Index.[31]
- The value of +7.7° (c = 4.0, CHCl3) for synthetic S-(+)-coniine and -7.9° (c = 0.5, CHCl3) for synthetic R-(−)-coniine is given by other chemists.[32]
Salts of given enantiomers do not necessarily have the same specific rotation as the same enantiomer of the free base. The hydrochloride salts of the (S)-(+) and (R)-(−) enantiomers of coniine have values of [α]D of +4.6° and -5.2°, respectively (c = 0.5, in methanol).[18]
Synthesis
Many syntheses of coniine have been reported over the last 50 years; one example of a stereoselective synthesis is that of Enders and Tiebes, who cite some of the earlier preparations.[32]
In the original synthesis of this substance by Ladenburg in 1886,[33] N-methylpyridine, as its iodide salt, was isomerised at 250 °C to obtain 2-methylpyridine (α-picoline). Reaction of this, as shown in the scheme below, with the cyclic trimer of acetaldehyde, paraldehyde, in the presence of a base gave 2-propenylpyridine via a Knoevenagel condensation. This intermediate was reduced with metallic sodium in ethanol or by hydrogen gas (as shown) to provide racemic (±) coniine. Enantiopure coniine was then obtained by a chiral resolution, specifically, fractional crystallisation of the diastereomeric (+)-tartaric acid salt.
The initial reaction, however, gives a poor yield and was improved by interaction of the two reagents at 150 °C in sealed tubes to give methyl-2-picolylalkyne, which was then heated at 185 °C with hydrochloric acid for 10 hours, producing a mixture of 2-propenylpyridine and 2-chloropropylpyridine. This mixture was reduced to rac-coniine by sodium in ethanol.
[The final structure in the above reaction scheme is drawn as that of a single enantiomer, although the final reaction produces a racemic product.]
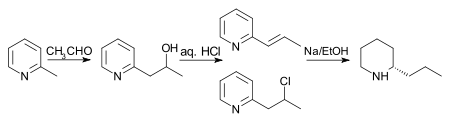
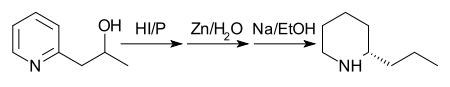
In 1907 the process was still further improved by reducing 2-(2'-hydroxypropyl)pyridine with phosphorus and hydroiodic acid at 125 °C and treating the product with zinc dust and water, then reducing the product with sodium in ethanol.[34]
[The final structure in the above reaction scheme is drawn as that of a single enantiomer, although the final reaction produces a racemic product.]
A number of other syntheses of coniine have been effected, of which that of Diels and Alder is of special interest.[35] The initial adduct of pyridine and dimethyl acetylenedicarboxylate is tetramethylquinolizine-1,2,3,4-tetracarboxylate, which on oxidation with dilute nitric acid is converted into trimethyl indolizine-tricarboxylate. This, on hydrolysis and decarboxylation, furnishes indolizine, the octahydro-derivate of which, also known as octahydropyrrocoline[36] is converted by the cyanogen bromide method successively into the bromocyanoamide, cyanoamide and rac.-coniine. A synthesis of the alkaloid, starting from indolizine (pyrrocoline) is described by Ochiai and Tsuda.[37]
[The final structure in the above reaction scheme is drawn as that of a single enantiomer, although the final reaction produces a racemic product.]
The preparation of L-(R)-coniine by the reduction of β-coniceine (L-propenylpiperidine) by Löffler and Friedrich[24] is interesting as a means of converting conhydrine to L-(R)-coniine. Hess and Eichel reported,[38] incorrectly,[39] that pelletierine was the aldehyde (β-2-piperidyl-propaldehyde) corresponding to coniine, and yielded rac-coniine when its hydrazone was heated with sodium ethoxide in ethanol at 156–170 °C. According to these authors, D-(S)-coniine is rendered almost optically inactive when heated with barium hydroxide and alcohol at 180–230 °C. Leithe[40] has shown by observation of the optical rotation of (+)-pipecolic acid (piperidine-2-carboxylic acid) and some of its derivatives under varying conditions,[41] that it must belong to the D-series of amino acids. Since (+)-conhydrine can be oxidised to (−)-pipecolic acid,[42] and transformed through β-coniceine into L-(R)-(−)-coniine,[24] it follows that (+)-coniine, (+)-2-methylpiperidine (α-pipecoline) and (+)-piperidine-2-carboxylic acid must all have similar spatial configurations.
Biosynthesis of Coniine
The complete biosynthesis of coniine is still being investigated. While the exact mechanism is still to be determined, much of the pathway has been elucidated.
Originally thought to use 4 acetyl groups as feed compounds for the polyketide synthase that forms coniine,[43] recent work[44] has led to the conclusion that two malonyl and a butyryl CoA are what is coupled together before further operations are performed to finally form coniine.
Initially, acetate is converted to acetyl-CoA, some of which is also used to form malonyl CoA. An acetyl CoA is further elongated using malonyl-CoA by fatty acid synthase to form butyryl-CoA.
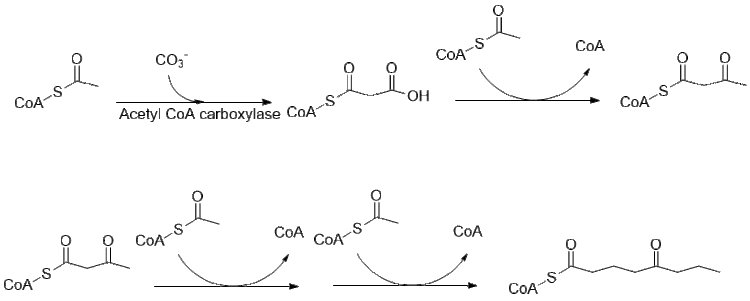
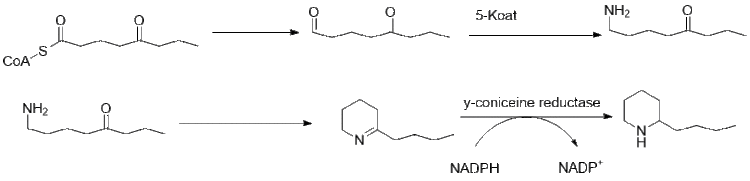
Further elongation of butyryl-CoA using 2 malonyl-CoA forms 5-ketooctanal. Ketooctanal then undergoes transamination using alanine:5-keto-octanal aminotransferase.[45] The amine then spontaneously cyclizes and is dehydrated to form the coniine precursor γ–coniceine. This is then reduced using NADPH dependent y-coniceine reductase to form coniine.
In popular culture
Coniine is the murder weapon in Agatha Christie's mystery novel Five Little Pigs.
References
- 1 2 DNRP-WLRD-RRS Staff (November 28, 2016). "Poison-hemlock". Noxious Weeds in King County, Weed Identification Photos. Seattle, WA: Department of Natural Resources and Parks (DNRP), Water and Land Resources Division (WLRD), Rural and Regional Services (RRS) section. Retrieved January 23, 2017.
- ↑ Peters, Amy; Bouska, Cassie. "Poison Hemlock". OSU Extension Service. Oregon State University. Retrieved 3 May 2015.
- ↑ Cheeke, Peter (31 Aug 1989). Toxicants of Plant Origin: Alkaloids, Volume 1 (1 ed.). Boca Raton, Florida: CRC Press. p. 118. ISBN 0849369908.
- ↑ "Poison Hemlock: Options for Control" (PDF). co.lincoln.wa.us. Lincoln County Noxious Weed Control Board. Retrieved 3 May 2015.
- ↑ "Poison Hemlock". pierecountryweedboard.wsu.edu. Pierce County Noxious Weed Control Board.
- ↑ "Poison Hemlock" (PDF). store.msuextension.org. Montana State University. Retrieved 3 May 2015.
- ↑ Vetter, J (September 2004). "Poison Hemlock (Conium maculatum L.)". Food Chem Toxicol. 42 (9): 1374–82. doi:10.1016/j.fct.2004.04.009. PMID 15234067.
- 1 2 Moser, L; Crisp, D. "Poison Hemlock" (PDF). San Francisco Peaks Weed Management. Retrieved 3 May 2015.
- ↑ N. V. Mody; R. Henson; P. A. Hedin; U. Kokpol; D. H. Miles (1976). "Isolation of the insect paralyzing agent coniine from Sarracenia flava". Experientia. 32 (7): 829–830. doi:10.1007/BF02003710.
- ↑ Mackie, Robin. "Yellow Pitcher Plant or Trumpets". United States Department of Agriculture. United States Department of Agriculture Forest Service. Retrieved 3 May 2015.
- ↑ Clapham, Tutin, & Warburg: Flora of the British Isles, 2nd edition, page 524.
- ↑

- ↑ James Warren (2001). "Socratic suicide". Journal of Hellenic Studies. 121: 91–106. doi:10.2307/631830. PMID 19681231.
- ↑ R. G. Frey (1978). "Did Socrates commit suicide?". Philosophy. 53 (203): 106–108. doi:10.1017/S0031819100016375.
- ↑

SOCRATES, son of the statuary Sophroniscus and of the midwife Phaenarete, was born at Athens, not earlier than 471 nor later than May or June 469 B.C. ... In 399, four years after the restoration and the amnesty, he was indicted as an offender against public morality. ... The accusation ran thus: "Socrates is guilty, firstly, of denying the gods recognized by the state and introducing new divinities, and, secondly, of corrupting the young." ... Under ordinary circumstances the condemned criminal drank the cup of hemlock on the day after the trial; but in the case of Socrates the rule that during the absence of the sacred ship sent annually to Delos no one should be put to death caused an exceptional
- ↑ "The Suicide of Socrates". EyeWitness to History. Retrieved 3 May 2015.
- ↑ Grieve, M. (1971). A Modern Herbal (2nd ed.). Mineola, N.Y.: Dover Publications. p. 392. ISBN 0-486-22798-7. Retrieved 3 May 2015.
- 1 2 3 4 5 Stephen T. Lee; Benedict T. Green; Kevin D. Welch; James A. Pfister; Kip E. Panter (2008). "Stereoselective potencies and relative toxicities of coniine enantiomers". Chemical Research in Toxicology. 21 (10): 2061–2064. doi:10.1021/tx800229w.
- ↑ Giseke, Aug. Lud. (1827) "Ueber das wirksame Princip des Schierlings, Conium maculatum" (On the active component of hemlock, Conium maculatum), Archiv der Pharmazie, 20 (2) : 97–111. On p. 99, Giseke credits the Swiss apothecary Peschier with coining the name coniin (coniine). See footnote on p. 87 of: Peschier (1821) "Neue analytische Untersuchungen über den unter verschiedenen Himmelsstrichen gebauten Mohn; ferner über einige inländische Narcotica, und Entdeckung neuer Pflanzensäuren und Alkälien in denselben" (New analytical investigations into poppies grown in various climates; furthermore, on some domestic narcotics, and discovery of new plant acids and alkalis in the same), Neues Journal der Pharmacie für Aerzte, Apotheker und Chemiker, 5 (1) : 76–101. From p. 87: "Eine Abbildung der krystallisirten Säure s. Fig. 1 das coniumsaure Natron ist Fig. 2 abgebildet." (An illustration of the crystalline acid, see Fig. 1 ; the sodium salt of conium acid is depicted in Fig. 2.)
- ↑ Blyth, J. (1849) "On the composition of coniine, and its products of decomposition," Quarterly Journal of the Chemical Society of London, 1 : 345–363. Blyth found the empirical formula of coniine to be (p. 351): C17H17N. The error in the amount of carbon is due, in part, to his having assumed that the atomic mass of carbon is 6, not 12 — a common error at the time.
- ↑ Hoffmann, A. W. (1881) "Einwirkung der Wärme auf die Ammoniumbasen: 2. Coniin" (Effect of heat on ammonium bases: 2. Coniine), Berichte der deutschen chemischen Gesellschaft, 14 : 705–713.
- ↑ Panter, K. E. and Keeler, R. F., Ch. 5: Piperidine alkaloids of poison hemlock (Conium maculatum) in: Cheeke, Peter R., ed., Toxicants of Plant Origin: Alkaloids, vol. 1 (Boca Raton, Florida: CRC Press, Inc., 1989), p. 116.
- ↑ Ahrens, Ber., 1902, 35, 1330
- 1 2 3 Löffler and Friedrich, Ber., 1909, 42, 107.
- ↑ Melzer, Arch. Pharm., 1898, 236, 701
- ↑ cf. Dilling, Pharm. J., 1909, [iv], 29, 34, 70, 102.
- ↑ Späth, Kuffner and Ensfellner, Ber., 1933, 66, 596.
- ↑ Gabutti, Chem. Soc. Abstr., 1906, [ii], 711.
- ↑ Craig J. Cymerman; A. R. Pinder (1971). "Improved method resolution of coniine". Journal of Organic Chemistry. 36 (23): 3648–3649. doi:10.1021/jo00822a051.
- ↑ A. Ladenburg (1888) Justus Liebig's Ann. Chem. 247 1-98.
- ↑ The Merck Index, 15th Ed. (2013), p. 446, Monograph 2489, O'Neil: The Royal Society of Chemistry. Available online at: http://www.rsc.org/Merck-Index/monograph/mono1500002489
- 1 2 D. Enders and J. Tiebes (1993) Liebig's Ann. Chem. 173-177.
- ↑ Chem. Soc. Abstr., 1886, 19, 439, 2579.
- ↑ Ber., 1907, 40, 3734
- ↑ Diels and Alder, Annalen, 1932, 498, 16.
- ↑ G. R. Clemo; G. R. Ramage (1932). "Octahydropyrrocoline". Journal of the Chemical Society: 2969–2973. doi:10.1039/JR9320002969.
- ↑ Ber., 1934, 67, 1011.
- ↑ Ber., 1917, 50, 1192, 1386.
- ↑ Pelletierine is now known to be 1-(2-piperidinyl)-2-propanone; see: The Merck Index, 15th Ed. (2013), p. 1314, Monograph 7181, O'Neil: The Royal Society of Chemistry. Available online at: http://www.rsc.org/Merck-Index/monograph/mono1500007181
- ↑ Ber., 1932, 65, 927.
- ↑ George William Clough (1918). "The relationship between the optical rotatory powers and the relative configurations of optically active compounds. The influence of certain inorganic haloids on the optical rotatory powers of α-hydroxy-acids, α-amino-acids, and their derivatives". Journal of the Chemical Society, Transactions. 113: 526–554. doi:10.1039/CT9181300526.
- ↑ Willstätter, Ber., 1901, 34, 3166.
- ↑ Leete E (1964) Biosynthesis of the hemlock alkaloids. The incorporation of acetate-1-C14 into coniine and conhydrine. J Am Chem Soc 86, 2509–2513
- ↑ Hannu Hotti, Tuulikki Seppanen-Laakso, Mikko Arvas, Teemu H. Teeri and Heiko Rischer, Polyketide synthases from poison hemlock (Conium maculatum L.) FEBS Journal 282, 2015 4141–4156
- ↑ Roberts MF Phytochemistry 17 1978, 107-112
Further reading
- Green, Benedict T.; Lee, Stephen T.; Panter, Kip E. & Brown, David R. (2012). "Piperidine Alkaloids: Human and Food Animal Teratogens" (PDF). Food and Chemical Toxicology. 50: 2049–2055. doi:10.1016/j.fct.2012.03.049. Retrieved January 23, 2017.