Chymopapain
| Chymopapain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
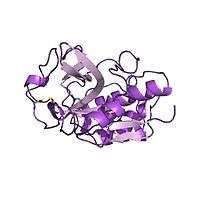 Chymopapain's structure | |||||||||
| Identifiers | |||||||||
| EC number | 3.4.22.6 | ||||||||
| CAS number | 2593837 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
| Clinical data | |
|---|---|
| Routes of administration | Injection into intervertebral disc |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| | |
Chymopapain (EC 3.4.22.6, chymopapain A, chymopapain B, chymopapain S, brand name Chymodiactin) is a proteolytic enzyme isolated from the latex of papaya (Carica papaya). It is a medication used to treat herniated lower lumbar discs in the spine.[1] Chymopapain injections are normally given under local, rather than general, anaesthesia. The dose for a single intervertebral disc is 2 to 4 nanokatals, with a maximum dose per patient of 8 nanokatals. The procedure is referred to as chemonucleolysis, a type of percutaneous discectomy.
The sale and distribution of chymopapain was discontinued in the United States on January 27, 2003 after the company producing it decided to stop selling worldwide.[2][3]
Structure
Primary structure
Chymopapain's zymogen is made up of a total of 352 residues, and it has a weight of approximately 23.78kDa. Three different regions can be distinguished inside the precursor's chain.[4]
- The first 18 aminoacids act as a sorting signal by indicating the final destination of chymopapain inside the cell when being sorted by the Golgi apparatus.[4] Although this final destination is not fully studied yet, other PLCPs are contained in lysosomes and other acidified vesicles and chymopapain is believed to be in these same vesicles as well.[5][6] Chymopapain is also known to be secreted outside the cell.[7]
- The second region is constituted by residues 19 to 134, which conform a propeptide that will be removed upon activation once chymopapain reaches its final destination inside the cell.[4] This region allows the protein to be properly folded in the endoplasmatic reticulum and to stabilize the chain in low pH conditions, which also supports the idea that chymopapain can be found in lysosomes.[5][6] The propeptide is folded in a way that prevents substrates from entering into the active site, thus blocking proteolytic activity until it is cleaved.[8][9]
- The rest of the protein -residues 135 to 352- conform chymopapain's mature chain.[4][4] Three aminoacids can be highlighted in this region, which are Cys159, His293 and Asn313, as they constitute the catalytic tryad of the enzyme.[10] Cys159 and His293 are the two residues that perform the catalysis of the substrate while Asn313 interacts with Cys159 and properly orients its imidazolium ring to allow the reaction to happen, thus bearing an essential function in the catalysis too.[11]
Secondary and tertiary structures
Chymopapain's structure was solved by X-ray diffraction techniques.[4] Analysis of this structure showed chymopapain to have 7 alpha helix regions, 10 beta sheet regions and 2 loop turns.[4] These 2 turns are the main difference between chymopapain's structure and other papaya proteinase proteins such as papain or caricain, which have similar conformations. [12][13]
Besides, chymopapain presents 3 disulfide bonds as post-traducional modifications stablished between residues 156-197, 190-229 and 287-338. [4]
Quaternary structure
Chymopapain presents a quaternary structure characterized by the formation of homo dimers, which means that two chymopapain chains join each other through weak interactions to conform one unique biological structure. [14]
Function
As well as all the other enzymes in the PLCPs group, chymopapain is a cysteine protease. Proteases are enzymes that hydrolyse peptide bond between the residues that conform a protein. In every hydrolysis a water molecule is released. Specifically, a cysteine protease is an enzyme which breaks the peptide bond by using the thiol group of a cysteine residue as the nucleophile. In order to hydrolyse, the whole catalytic triad of the enzyme must be used. This is constituted by a cysteine, a histidine and a third residue, which tens to be a asparagine. The functional groups used in the reaction are the thiol group of the cysteine and the imidazolium ring of a histidine.
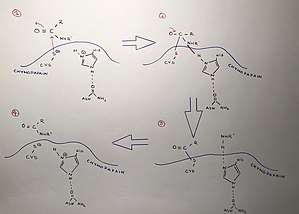
The mechanism followed is exposed below:
- The thiol group from the cysteine loses a proton, so it becomes negative charged and the amino group of the histidine catches a proton, which gives it a positive charge.
- The cysteine makes a bond with the carbon breaking the carbon's double bond with oxygen and converting it into a simple bond.
- The amino group is attracted by the positive charge of the histidine and a bond between these two is formed. The peptide bond is now broken and the carbonyl group is remade.
- The NH2R group is released from the histidine. The bond between the thiol group from the cysteine and the carbon is broken and a NHR group replaces it.
When this two bonds are broken, the catalytic triad from the chymopapain is available to be used again.
Medical applications
Chymopapain is one of the substract uses in chemonucleolysis.[15] This method was a new proposal to treat primary lumbar intervertebral disc disease by nonsurgical method. Purified chymopapain is the main component of the injure, composed basically of 20 mg in five millilitres. It is provided in vials containing 10.000 units of the lyophilized agent with 0.37 mg of disodium edetate,[16] 3.5 mg of cysteine hydrochloride monohydrate and 1.0 mg of bisulfide. All of them work as stabilizers and activators. Sodium hydroxide is in charge of adjust the PH of the solution. Yhen the injection in rehydrate with 5 milillitres of steril water.
A surgeon inject the solution directly into the herniated disk on the spine to dissolve part of it and ease the pain. The process is under fluoroscopic control. Chymopapain is the responsible for catalyse, both in vivo and in vitro, a rapid reduction in the viscosity and, as a consequence, the weight of the nucleus pulposus. In fact, it is a depolymerization of the chondromuchoprotein[17] and a decrease in the abily of a disk to imbibe fluid.
This enzyme has been studied by important deparments of universities around the world. It was providing as much in animals as in humans and very rarely it caused seriou side effects including paralysis of the legs and death[18]. Also, it could cause anaphylaxis, but only in 1% of the patients who recieve the medication.
See also
References
- ↑ Chymopapain Injection from MedicineNet
- ↑ http://www.mayoclinic.com/health/drug-information/DR600373
- ↑ The Current Status of Chymopapain
- 1 2 3 4 5 6 7 8 Maes D, Bouckaert J, Poortmans F, Wyns L, Looze Y (December 1996). "Structure of chymopapain at 1.7 A resolution". Biochemistry. 35 (50): 16292–8. doi:10.1021/bi961491w. PMID 8973203.
- 1 2 EMBL-EBI, InterPro. "Peptidase C1A, papain C-terminal (IPR000668) < InterPro < EMBL-EBI". www.ebi.ac.uk. Retrieved 2018-10-11.
- 1 2 group, NIH/NLM/NCBI/IEB/CDD. "NCBI CDD Conserved Protein Domain Peptidase_C1A". www.ncbi.nlm.nih.gov. Retrieved 2018-10-11.
- ↑ Oberg KA, Ruysschaert JM, Azarkan M, Smolders N, Zerhouni S, Wintjens R, Amrani A, Looze Y (November 1998). "Papaya glutamine cyclase, a plant enzyme highly resistant to proteolysis, adopts an all-beta conformation". European Journal of Biochemistry. 258 (1): 214–22. doi:10.1046/j.1432-1327.1998.2580214.x. PMID 9851712.
- ↑ "Chymopapain - Carica papaya (Papaya)". www.uniprot.org. Retrieved 2018-10-11.
- ↑ "SMART: Inhibitor_I29 domain annotation". smart.embl.de. Retrieved 2018-10-11.
- ↑ Gariev IA. "HCS: Chymopapain". www.enzyme.chem.msu.ru. Retrieved 2018-10-11.
- ↑ López-Iglesias M, Gotor-Fernández V (August 2015). "Recent Advances in Biocatalytic Promiscuity: Hydrolase-Catalyzed Reactions for Nonconventional Transformations". Chemical Record. 15 (4): 743–59. doi:10.1002/tcr.201500008. PMID 26147872.
- ↑ Jacquet A, Kleinschmidt T, Schnek AG, Looze Y, Braunitzer G (May 1989). "The thiol proteinases from the latex of Carica papaya L. III. The primary structure of chymopapain". Biological Chemistry Hoppe-Seyler. 370 (5): 425–34. PMID 2500950.
- ↑ Watson DC, Yaguchi M, Lynn KR (February 1990). "The amino acid sequence of chymopapain from Carica papaya". The Biochemical Journal. 266 (1): 75–81. PMC 1131098. PMID 2106878.
- ↑ "HotSpot Wizard 3.0". loschmidt.chemi.muni.cz. Retrieved 2018-10-11.
- ↑ "Chemonycleolysis" (PDF).
- ↑ "Edetate disodium".
- ↑ "Description of chondromucoprotein".
- ↑ "Chymopapain". The Mayo Clinic.
Further reading
- The MEROPS online database for peptidases and their inhibitors: C01.002
- Data sheet for Papain from BIOZYM