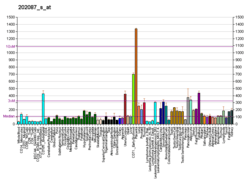
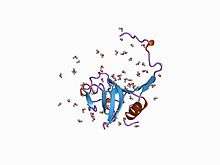
Cathepsin L1
Cathepsin L1 is a protein that in humans is encoded by the CTSL1 gene.[3][4][5]
The protein encoded by this gene is a lysosomal cysteine protease that plays a major role in intracellular protein catabolism. Its substrates include collagen and elastin, as well as alpha-1 protease inhibitor, a major controlling element of neutrophil elastase activity. The encoded protein has been implicated in several pathologic processes, including myofibril necrosis in myopathies and in myocardial ischemia, and in the renal tubular response to proteinuria. This protein, which is a member of the peptidase C1 family, is a dimer composed of disulfide-linked heavy and light chains, both produced from a single protein precursor. At least two transcript variants encoding the same protein have been found for this gene.[5]
Interactions
CTSL1 has been shown to interact with Cystatin A.[6][7]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000135047 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ Chauhan SS, Popescu NC, Ray D, Fleischmann R, Gottesman MM, Troen BR (Feb 1993). "Cloning, genomic organization, and chromosomal localization of human cathepsin L". J Biol Chem. 268 (2): 1039–45. PMID 8419312.
- ↑ Joseph LJ, Chang LC, Stamenkovich D, Sukhatme VP (Jun 1988). "Complete nucleotide and deduced amino acid sequences of human and murine preprocathepsin L. An abundant transcript induced by transformation of fibroblasts". J Clin Invest. 81 (5): 1621–9. doi:10.1172/JCI113497. PMC 442598. PMID 2835398.
- 1 2 "Entrez Gene: CTSL1 cathepsin L1".
- ↑ Majerle, Andreja; Jerala Roman (Sep 2003). "Protein inhibitors form complexes with procathepsin L and augment cleavage of the propeptide". Arch. Biochem. Biophys. United States. 417 (1): 53–8. doi:10.1016/S0003-9861(03)00319-9. ISSN 0003-9861. PMID 12921779.
- ↑ Estrada, S; Nycander M; Hill N J; Craven C J; Waltho J P; Björk I (May 1998). "The role of Gly-4 of human cystatin A (stefin A) in the binding of target proteinases. Characterization by kinetic and equilibrium methods of the interactions of cystatin A Gly-4 mutants with papain, cathepsin B, and cathepsin L". Biochemistry. UNITED STATES. 37 (20): 7551–60. doi:10.1021/bi980026r. ISSN 0006-2960. PMID 9585570.
Further reading
- Smith CG, Smith MT, Besch NF, et al. (1980). "Effect of delta 9-tetrahydrocannabinol (THC) on female reproductive function". Advances in the biosciences. 22-23: 449–67. PMID 116880.
- Goretzki L, Schmitt M, Mann K, et al. (1992). "Effective activation of the proenzyme form of the urokinase-type plasminogen activator (pro-uPA) by the cysteine protease cathepsin L.". FEBS Lett. 297 (1–2): 112–8. doi:10.1016/0014-5793(92)80339-I. PMID 1551416.
- Dunn AD, Crutchfield HE, Dunn JT (1991). "Thyroglobulin processing by thyroidal proteases. Major sites of cleavage by cathepsins B, D, and L.". J. Biol. Chem. 266 (30): 20198–204. PMID 1939080.
- Stearns NA, Dong JM, Pan JX, et al. (1991). "Comparison of cathepsin L synthesized by normal and transformed cells at the gene, message, protein, and oligosaccharide levels". Arch. Biochem. Biophys. 283 (2): 447–57. doi:10.1016/0003-9861(90)90666-M. PMID 2275556.
- Ritonja A, Popović T, Kotnik M, et al. (1988). "Amino acid sequences of the human kidney cathepsins H and L.". FEBS Lett. 228 (2): 341–5. doi:10.1016/0014-5793(88)80028-0. PMID 3342889.
- Gal S, Gottesman MM (1988). "Isolation and sequence of a cDNA for human pro-(cathepsin L)". Biochem. J. 253 (1): 303–6. PMC 1149292. PMID 3421948.
- Johnson DA, Barrett AJ, Mason RW (1986). "Cathepsin L inactivates alpha 1-proteinase inhibitor by cleavage in the reactive site region". J. Biol. Chem. 261 (31): 14748–51. PMID 3490478.
- Mason RW, Walker JE, Northrop FD (1987). "The N-terminal amino acid sequences of the heavy and light chains of human cathepsin L. Relationship to a cDNA clone for a major cysteine proteinase from a mouse macrophage cell line". Biochem. J. 240 (2): 373–7. PMC 1147428. PMID 3545185.
- Joseph L, Lapid S, Sukhatme V (1987). "The major ras induced protein in NIH3T3 cells is cathepsin L." Nucleic Acids Res. 15 (7): 3186. doi:10.1093/nar/15.7.3186. PMC 340927. PMID 3550705.
- Kärgel HJ, Dettmer R, Etzold G, et al. (1982). "Action of rat liver cathepsin L on glucagon". Acta Biol. Med. Ger. 40 (9): 1139–43. PMID 7340337.
- Bevec T, Stoka V, Pungercic G, et al. (1996). "Major histocompatibility complex class II-associated p41 invariant chain fragment is a strong inhibitor of lysosomal cathepsin L." J. Exp. Med. 183 (4): 1331–8. doi:10.1084/jem.183.4.1331. PMC 2192513. PMID 8666891.
- Coulombe R, Grochulski P, Sivaraman J, et al. (1996). "Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment". EMBO J. 15 (20): 5492–503. PMC 452294. PMID 8896443.
- Baumgrass R, Williamson MK, Price PA (1997). "Identification of peptide fragments generated by digestion of bovine and human osteocalcin with the lysosomal proteinases cathepsin B, D, L, H, and S.". J. Bone Miner. Res. 12 (3): 447–55. doi:10.1359/jbmr.1997.12.3.447. PMID 9076588.
- Fujishima A, Imai Y, Nomura T, et al. (1997). "The crystal structure of human cathepsin L complexed with E-64". FEBS Lett. 407 (1): 47–50. doi:10.1016/S0014-5793(97)00216-0. PMID 9141479.
- Ménard R, Carmona E, Takebe S, et al. (1998). "Autocatalytic processing of recombinant human procathepsin L. Contribution of both intermolecular and unimolecular events in the processing of procathepsin L in vitro". J. Biol. Chem. 273 (8): 4478–84. doi:10.1074/jbc.273.8.4478. PMID 9468501.
- Schick C, Pemberton PA, Shi GP, et al. (1998). "Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: a kinetic analysis". Biochemistry. 37 (15): 5258–66. doi:10.1021/bi972521d. PMID 9548757.
- Estrada S, Nycander M, Hill NJ, et al. (1998). "The role of Gly-4 of human cystatin A (stefin A) in the binding of target proteinases. Characterization by kinetic and equilibrium methods of the interactions of cystatin A Gly-4 mutants with papain, cathepsin B, and cathepsin L.". Biochemistry. 37 (20): 7551–60. doi:10.1021/bi980026r. PMID 9585570.
- Halfon S, Ford J, Foster J, et al. (1998). "Leukocystatin, a new Class II cystatin expressed selectively by hematopoietic cells". J. Biol. Chem. 273 (26): 16400–8. doi:10.1074/jbc.273.26.16400. PMID 9632704.
External links