Bunyavirales
| Bunyavirales | |
|---|---|
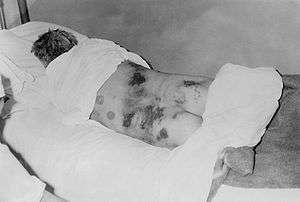 | |
| Isolated male diagnosed with Crimean-Congo hemorrhagic fever | |
| Virus classification | |
| Group: | Group V ((-)ssRNA) |
| Order: | Bunyavirales |
| Families | |
|
Hantaviridae | |
Bunyavirales is an order of negative-sense single-stranded RNA viruses. It was formerly known as Bunyaviridae family of viruses.
In 2017, the ICTV reclassified the family Bunyaviridae as Bunyavirales, a taxonomic shift from a family of viruses to an order of viruses.[1][2] The body made these decisions in a 2016 convening in Budapest.[2] Primary reasons for this alteration revolve around these observations: approximately half of viruses in the former Bunyaviridae were at the time unassigned to a genus; novel viruses discovered that were characteristic of and clustered around Bunyaviridae based on phylogenetic analyses had bi-segmented genomes (as opposed to Bunyaviridae's tri-segmentation); and plant viruses also lacking tri-segmentation were previously known to be "bunya-like" yet were not properly assigned to the family Bunyaviridae based upon the past taxonomic classifications. All five genera formerly in the family Bunyaviridae (Hantavirus, Nairovirus, Orthobunyavirus, Phlebovirus, Tospovirus) are now novel viral families, some of which have been combined. These new families include: Hantaviridae, Feraviridae, Fimoviridae, Jonviridae, Nairoviridae, Peribunyaviridae, Phasmaviridae, Phenuiviridae, and Tospoviridae.
This order of viruses belong to the fifth group of the Baltimore classification, the so-called negative-sense single stranded ribonucleic acid (−)ssRNA. They are enveloped RNA viruses. Though generally found in arthropods or rodents, certain viruses in this order occasionally infect humans. Some of them also infect plants.[3]
A majority of bunyaviruses are vector-borne. With the exception of Hantaviruses, all viruses in the Bunyavirales order are transmitted by arthropods (mosquitos, tick, or sandfly). Hantaviruses are transmitted through contact with deer mice feces. Incidence of infection is closely linked to vector activity, for example, mosquito-borne viruses are more common in the summer.[3]
Human infections with certain members of Bunyavirales, such as Crimean-Congo hemorrhagic fever virus, are associated with high levels of morbidity and mortality, consequently handling of these viruses must occur with a Biosafety level 4 laboratory. They are also the cause of severe fever with thrombocytopenia syndrome.[4]
Hanta virus or Hantavirus Hemorrhagic fever, common in Korea, Scandinavia, Russia, and western North America, is associated with high fever, lung edema and pulmonary failure. Mortality is around 55%. The antibody reaction plays an important role in decreasing levels of viremia.
Virology
Classification
There are currently about 330 viruses recognised in this order.
The order Bunyavirales contains a number of families:
- Family Hantaviridae; type species: Hantaan virus
- Family Nairoviridae; type species: Dugbe virus
- Family Peribunyaviridae (which contains former genus Orthobunyavirus); type species: Bunyamwera virus
- Family Phenuiviridae (which contains former genus Phlebovirus; type species: Rift Valley fever virus
- Family Tospoviridae; type species: Tomato spotted wilt virus
- Family Feraviridae; type species: Ferak Virus
- Family Fimoviridae;[5] type species: Fig mosaic emaravirus
- Family Jonviridae; type species: Jonchet orthojonvirus
- Family Phasmaviridae; type species: Kigluaik phantom orthophasmavirus
Plants can host Bunyaviruses from the Tospoviridae and Fimoviridae families (tomato, pigeonpea, melon, wheat, raspberry, redbud, rose).
There are a number of viruses that have not yet been placed in a genus: these include Gan Gan virus, Maprik virus, Mapputta virus and Trubanaman virus.
Members of some families appear to be insect-specific, e.g. the phasmavirids, first isolated from phantom midges,[6] and since identified in diverse insects including moths, wasps and bees, and other true flies.
Structure
Bunyavirus morphology is somewhat similar to that of the Paramyxoviridae family; Bunyavirales form enveloped, spherical virions with diameters of 90–100 nm. These viruses contain no matrix proteins.
Genome
Bunyaviridae have tripartite genomes consisting of a large (L), medium (M), and small (S) RNA segment. These RNA segments are single-stranded, and exist in a helical formation within the virion. Besides, they exhibit a pseudo-circular structure due to each segment's complementary ends. The L segment encodes the RNA Dependent RNA-polymerase, necessary for viral RNA replication and mRNA synthesis. The M segment encodes the viral glycoproteins, which project from the viral surface and aid the virus in attaching to and entering the host cell. The S segment encodes the nucleocapsid protein (N).[7]
The L and M segment are negative sense. For the Genera of Phlebovirus and Tospovirus, the S segment is ambisense. Ambisense means that some of the genes on the RNA strand are negative sense and others are positive sense. The S segment codes for the viral nucleoprotein (N) in the negative sense and a nonstructural (NSs) protein in ambisense.
Replication
This ambisense arrangement requires two rounds of transcription to be carried out. First the negative sense RNA is transcribed to produce mRNA and a full length replicative intermediate. From this intermediate a subgenomic mRNA encoding the small segment nonstructural protein is produced while the polymerase produced following the first round of transcription can now replicate the full length RNA to produce viral genomes.
Bunyavirus RNA replicates in the cytoplasm, while the viral proteins transit through the ER and Golgi apparatus. Mature virions bud from the Golgi apparatus into vesicles which are transported to the cell surface.
Diseases in humans
Bunyaviruses that cause disease in humans include:
- California encephalitis virus, La Crosse encephalitis virus, Jamestown Canyon virus and Snowshoe hare virus vector: mosquitoes Family: Peribunyaviridae
- Hantavirus reservoir: small mammals or rodents vector: aerosolized excreta from these mammals Family: Hantaviridae
- Crimean–Congo hemorrhagic fever reservoir and vector: ticks, amplifying hosts and vector: small mammals, domestic mammals Family Nairoviridae
- Rift Valley fever reservoir: bats vector: mosquitoes amplifying hosts: small mammals, domestic mammals Family: Phenuiviridae
- Bwamba Fever reservoir: monkeys vector: mosquitoes, amplifying hosts: donkeys Family: Peribunyaviridae
- Severe fever with thrombocytopenia syndrome
Bunyaviruses have segmented genomes, making them capable of rapid recombination and increasing the risk of outbreak.[9] Bunyaviridae are transmitted by hematophagous arthropods including mosquitoes, midges, flies, and ticks. The viral incubation period is about 48 hours. Symptomatic infection typically causes non-specific flu-like symptoms with fever lasting for about three days. Because of their non-specific symptoms, Bunyavirus infections are frequently mistaken for other illnesses. For example, Bwamba fever is often mistaken for malaria.[10]
Prevention
Prevention depends on the reservoir, amplifying hosts and how the viruses are transmitted, i.e. the vector, whether ticks or mosquitoes and which animals are involved.
Preventative measures include general hygiene, limiting contact with vector saliva, urine, feces, or bedding.
There are no licensed vaccine for bunyaviruses.
As precautions Cache Valley virus and Hantavirus research should be conducted in BSL-2 (or higher), Rift Valley Fever virus research be conducted in BSL-3 (or higher), Congo-Crimean Hemorrhagic Fever virus research be conducted in BSL-4 laboratories, per CDC.
Timeline
1940´s: Crimean–Congo hemorrhagic fever is discovered in Russia
1951: 3,000 cases of Hantavirus were reported in South Korea in 1951, a time when UN forces were fighting on the 38th parallel during the Korean War
1956: Cache Valley virus isolated in Culiseta inornata mosquitoes in Utah
1960: La Crosse virus was first recognized in a fatal case of encephalitis in La Crosse, Wisconsin
1977: Rift Valley Fever virus caused approximately 200,000 cases and 598 deaths in Egypt
2017: Bunyavirales order is created
References
- ↑ Adams, Michael J.; Lefkowitz, Elliot J.; King, Andrew M. Q.; Harrach, Balázs; Harrison, Robert L.; Knowles, Nick J.; Kropinski, Andrew M.; Krupovic, Mart; Kuhn, Jens H. (2017-08-01). "Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017)". Archives of Virology. 162 (8): 2505–2538. doi:10.1007/s00705-017-3358-5. ISSN 0304-8608. PMID 28434098.
- 1 2 "Taxonomy". International Committee on Taxonomy of Viruses (ICTV). Retrieved 2017-09-29.
- 1 2 Plyusnin, A; Elliott, RM, eds. (2011). Bunyaviridae: Molecular and Cellular Biology. Caister Academic Press. ISBN 978-1-904455-90-5.
- ↑ Yu XJ, Liang MF, Zhang SY, et al. (April 2011). "Fever with thrombocytopenia associated with a novel bunyavirus in China". N. Engl. J. Med. 364 (16): 1523–32. doi:10.1056/NEJMoa1010095. PMC 3113718. PMID 21410387.
- ↑ "viralzone.expasy.org/7078?outline=all_by_species". viralzone.expasy.org. Retrieved 2017-09-29.
- ↑ Ballinger, MJ; Bruenn, JA; Hay, J; Czechowski, D; Taylor, DJ (2014). "Discovery and evolution of bunyavirids in arctic phantom midges and ancient bunyavirid-like sequences in insect genomes". J Virol.
- ↑ Ariza, A.; Tanner, S. J.; Walter, C. T.; Dent, K. C.; Shepherd, D. A.; Wu, W.; Matthews, S. V.; Hiscox, J. A.; Green, T. J. (2013-06-01). "Nucleocapsid protein structures from orthobunyaviruses reveal insight into ribonucleoprotein architecture and RNA polymerization". Nucleic Acids Research. 41 (11): 5912–5926. doi:10.1093/nar/gkt268. ISSN 0305-1048. PMC 3675483. PMID 23595147.
- ↑ "00.011. Bunyaviridae". ICTVdB—The Universal Virus Database, version 4. 2006. Retrieved 2009-01-01.
- ↑ Horne, Kate McElroy; Vanlandingham, Dana L. (2014-11-13). "Bunyavirus-Vector Interactions". Viruses. 6 (11): 4373–4397. doi:10.3390/v6114373. ISSN 1999-4915. PMC 4246228. PMID 25402172.
- ↑ Patrick R. Murray, Ken S. Rosenthal and Michael A. Pfaller (2008-12-24). Medical Microbiology, 6e (6 ed.). Philadelphia: Mosby. ISBN 9780323054706.
External links
- Viralzone: Bunyaviridae
- ICTVdb Index of Viruses—Bunyaviridae
- The Big Picture Book of Viruses: Bunyaviridae
- Bunyaviridae Genomes—database search results from the Viral Bioinformatics Resource Center
- Virus Pathogen Database and Analysis Resource (ViPR): Bunyaviridae
- "Bunyaviridae". NCBI Taxonomy Browser. 11571.