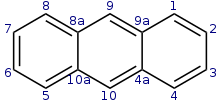
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.[13]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Anthracene | |
| Systematic IUPAC name
Tricyclo[8.4.0.03,8]tetradeca-1,3,5,7,9,11,13-heptaene | |
| Identifiers | |
3D model (JSmol) |
|
Beilstein Reference |
1905429 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.974 |
| EC Number |
|
Gmelin Reference |
67837 |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
| C14H10 | |
| Molar mass | 178.234 g·mol−1 |
| Appearance | Colorless |
| Odor | Weak aromatic |
| Density | 1.28 g/cm3 (25 °C)[1] 0.969 g/cm3 (220 °C) |
| Melting point | 216 °C (421 °F; 489 K)[1] at 760 mmHg |
| Boiling point | 341.3 °C (646.3 °F; 614.5 K)[1] at 760 mmHg |
| 0.022 mg/L (0 °C) 0.044 mg/L (25 °C) 0.29 mg/L (50 °C) 0.00045% w/w (100 °C, 3.9 MPa)[2] | |
| Solubility | Soluble in alcohol, (C2H5)2O, acetone, C6H6, CHCl3,[1] CS2[3] |
| Solubility in ethanol | 0.76 g/kg (16 °C) 1.9 g/kg (19.5 °C) 3.28 g/kg (25 °C)[3] |
| Solubility in methanol | 18 g/kg (19.5 °C)[3] |
| Solubility in hexane | 3.7 g/kg[3] |
| Solubility in toluene | 9.2 g/kg (16.5 °C) 129.4 g/kg (100 °C)[3] |
| Solubility in carbon tetrachloride | 7.32 g/kg[3] |
| log P | 4.56 |
| Vapor pressure | 0.01 kPa (125.9 °C) 0.1 kPa (151.5 °C)[4] 13.4 kPa (250 °C)[5] |
Henry's law constant (kH) |
0.0396 L·atm/mol[6] |
| UV-vis (λmax) | 345.6 nm, 363.2 nm[5] |
| −129.8×10−6 cm3/mol[7] | |
| Thermal conductivity | 0.1416 W/(m·K) (240 °C) 0.1334 W/(m·K) (270 °C) 0.1259 W/(m·K) (300 °C)[8] |
| Viscosity | 0.602 cP (240 °C) 0.498 cP (270 °C) 0.429 cP (300 °C)[8] |
| Structure | |
| Monoclinic (290 K)[9] | |
Space group |
P21/b[9] |
| D5 2h[9] | |
Lattice constant |
α = 90°, β = 124.7°, γ = 90° |
| Thermochemistry[10] | |
Heat capacity (C) |
210.5 J/(mol·K) |
Std molar entropy (S |
207.5 J/(mol·K) |
Std enthalpy of formation (ΔfH⦵298) |
129.2 kJ/mol |
Std enthalpy of combustion (ΔcH⦵298) |
7061 kJ/mol[5] |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335, H410[11] |
GHS precautionary statements |
P261, P273, P305+351+338, P501[11] |
| NFPA 704 (fire diamond) | 
1
2
1 |
| Flash point | 121 °C (250 °F; 394 K)[11] |
Autoignition temperature |
540 °C (1,004 °F; 813 K)[11] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
4900 mg/kg (rats, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence and production
Coal tar, which contains around 1.5% anthracene, remains a major source of this material. Common impurities are phenanthrene and carbazole. The mineral form of anthracene is called freitalite and is related to a coal deposit.[14] A classic laboratory method for the preparation of anthracene is by cyclodehydration of o-methyl- or o-methylene-substituted diarylketones in the so-called Elbs reaction.
Reactions
Reduction
Reduction of anthracene with alkali metals yields the deeply colored radical anion salts M+[anthracene]− (M = Li, Na, K). Hydrogenation gives 9,10-dihydroanthracene, preserving the aromaticity of the two flanking rings.
Cycloadditions
Anthracene photodimerizes by the action of UV light:
The dimer, called dianthracene (or sometimes paranthracene), is connected by a pair of new carbon-carbon bonds, the result of the [4+4] cycloaddition. It reverts to anthracene thermally or with UV irradiation below 300 nm. Substituted anthracene derivatives behave similarly. The reaction is affected by the presence of oxygen.[15][16]
Anthracene also reacts with dienophile singlet oxygen in a [4+2]-cycloaddition (Diels–Alder reaction):
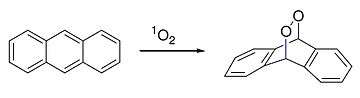 Diels alder reaction of anthracene with singlet oxygen
Diels alder reaction of anthracene with singlet oxygen
With electrophiles
Chemical oxidation occurs readily, giving anthraquinone, C14H8O2 (below), for example using hydrogen peroxide and vanadyl acetylacetonate.[17]
 Anthraquione
Anthraquione
Electrophilic substitution of anthracene occurs at the 9 position. For example, formylation affords 9-anthracenecarboxaldehyde. Substitution at other positions is effected indirectly, for example starting with anthroquinone.[18]
Uses
Anthracene is converted mainly to anthraquinone, a precursor to dyes.[19]
Niche
Anthracene, a wide band-gap organic semiconductor is used as a scintillator for detectors of high energy photons, electrons and alpha particles. Plastics, such as polyvinyltoluene, can be doped with anthracene to produce a plastic scintillator that is approximately water-equivalent for use in radiation therapy dosimetry. Anthracene's emission spectrum peaks at between 400 nm and 440 nm.
It is also used in wood preservatives, insecticides, and coating materials.
Anthracene is commonly used as a UV tracer in conformal coatings applied to printed wiring boards. The anthracene tracer allows the conformal coating to be inspected under UV light.[20] Anthracene also used in manufacturing of anthraquinone.
Derivatives

A variety of anthracene derivatives find specialized uses. Derivatives having a hydroxyl group are 1-hydroxyanthracene and 2-hydroxyanthracene, homologous to phenol and naphthols, and hydroxyanthracene (also called anthrol, and anthracenol)[21][22] are pharmacologically active. Anthracene may also be found with multiple hydroxyl groups, as in 9,10-dihydroxyanthracene.
Occurrence
Anthracene, as many other polycyclic aromatic hydrocarbons, is generated during combustion processes. Exposure to humans happens mainly through tobacco smoke and ingestion of food contaminated with combustion products.
Toxicology
Many investigations indicate that anthracene is noncarcinogenic: "consistently negative findings in numerous in vitro and in vivo genotoxicity tests". Early experiments suggested otherwise because crude samples were contaminated with other polycyclic aromatic compounds. Furthermore, it is readily biodegraded in soil. It is especially susceptible to degradation in the presence of light.[19]
See also
- 9,10-Dithioanthracene, derivative with two thiol groups added to the central ring
- Phenanthrene
- Tetracene
References
- Haynes, p. 3.28
- Haynes, p. 5.157
- Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. pp. 81.
- Haynes, p. 6.116
- Anthracene in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-06-22)
- Haynes, p. 5.157
- Haynes, p. 3.579
- "Properties of Anthracene". www.infotherm.com. Wiley Information Services GmbH. Archived from the original on 2014-11-01. Retrieved 2014-06-22.
- Douglas, Bodie E.; Ho, Shih-Ming (2007). Structure and Chemistry of Crystalline Solids. New York: Springer Science+Business Media, Inc. p. 289. ISBN 978-0-387-26147-8.
- Haynes, p. 5.41
- Sigma-Aldrich Co., Anthracene. Retrieved on 2014-06-22.
- "MSDS of Anthracene". www.fishersci.ca. Fisher Scientific. Retrieved 2014-06-22.
- Lindsey, Jonathan; et al. "Anthracene". PhotochemCAD. Retrieved 20 February 2014.
- Freitalite, Mindat, https://www.mindat.org/min-54360.html
- Rickborn, Bruce (1998). "The Retro-Diels-Alder Reaction Part I. C−C Dienophiles". Organic Reactions. pp. 1–393. doi:10.1002/0471264180.or052.01. ISBN 978-0471264187.
- Bouas-Laurent, Henri; Desvergne, Jean-Pierre; Castellan, Alain; Lapouyade, Rene (2000). "Photodimerization of anthracenes in fluid solution: Structural aspects". Chemical Society Reviews. 29: 43–55. doi:10.1039/a801821i.
- Charleton, Kimberly D. M.; Prokopchuk, Ernest M. (2011). "Coordination Complexes as Catalysts: The Oxidation of Anthracene by Hydrogen Peroxide in the Presence of VO(acac)2". Journal of Chemical Education. 88 (8): 1155–1157. Bibcode:2011JChEd..88.1155C. doi:10.1021/ed100843a.
- Škalamera, Đani; Veljković, Jelena; Ptiček, Lucija; Sambol, Matija; Mlinarić-Majerski, Kata; Basarić, Nikola (2017). "Synthesis of asymmetrically disubstituted anthracenes". Tetrahedron. 73 (40): 5892–5899. doi:10.1016/j.tet.2017.08.038.
- Collin, Gerd; Höke, Hartmut and Talbiersky, Jörg (2006) "Anthracene" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a02_343.pub2
- Zeitler, Alex (2012-06-27) Conformal Coating 101: General Overview, Process Development, and Control Methods. BTW, Inc.
- 1-Hydroxyanthracene. NIST datapage
- 2-Hydroxyanthracene. NIST datapage
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. ISBN 1439855110.
External links
| Wikimedia Commons has media related to Anthracenes. |
| Wikisource has the text of the 1879 American Cyclopædia article Anthracene. |
- Image of anthracene crystals
- International Chemical Safety Card 0825
- IARC – Monograph 32
- National Pollutant Inventory – Polycyclic Aromatic Hydrocarbon Fact Sheet
- European Chemicals Agency – ECHA
- . Encyclopædia Britannica (11th ed.). 1911.