Methane
Methane (US: /ˈmɛθeɪn/ or UK: /ˈmiːθeɪn/) is a chemical compound with the chemical formula CH4 (one atom of carbon and four atoms of hydrogen). It is a group-14 hydride and the simplest alkane, and is the main constituent of natural gas. The relative abundance of methane on Earth makes it an attractive fuel, although capturing and storing it poses challenges due to its gaseous state under normal conditions for temperature and pressure.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methane[1] | |||
| Systematic IUPAC name
Carbane (never recommended[1]) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | |||
Beilstein Reference |
1718732 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.739 | ||
| EC Number |
| ||
Gmelin Reference |
59 | ||
| KEGG | |||
| MeSH | Methane | ||
PubChem CID |
|||
| RTECS number |
| ||
| UN number | 1971 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
| |||
| Properties | |||
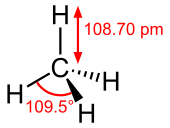
| CH4 | |||
| Molar mass | 16.043 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density |
| ||
| Melting point | −182.5 °C; −296.4 °F; 90.7 K | ||
| Boiling point | −161.50 °C; −258.70 °F; 111.65 K[3] | ||
| 22.7 mg·L−1 | |||
| Solubility | Soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone and insoluble in water | ||
| log P | 1.09 | ||
Henry's law constant (kH) |
14 nmol·Pa−1·kg−1 | ||
| Conjugate acid | Methanium | ||
| Conjugate base | Methyl anion | ||
| −12.2×10−6 cm3·mol−1 | |||
| Structure | |||
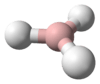
| Td | |||
Molecular shape |
Tetrahedron | ||
| 0 D | |||
| Thermochemistry | |||
Heat capacity (C) |
35.69 J·(K·mol)−1 | ||
Std molar entropy (S |
186.25 J·(K·mol)−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−74.87 kJ·mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−891.1 to −890.3 kJ·mol−1 | ||
| Hazards[4] | |||
| Safety data sheet | See: data page | ||
| GHS pictograms |  | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H220 | ||
GHS precautionary statements |
P210 | ||
| NFPA 704 (fire diamond) | 
4
2
0 SA | ||
| Flash point | −188 °C (−306.4 °F; 85.1 K) | ||
Autoignition temperature |
537 °C (999 °F; 810 K) | ||
| Explosive limits | 4.4–17% | ||
| Related compounds | |||
Related alkanes |
| ||
| Supplementary data page | |||
Structure and properties |
Refractive index (n), Dielectric constant (εr), etc. | ||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
Spectral data |
UV, IR, NMR, MS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Naturally occurring methane is found both below ground and under the seafloor, and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane.[6] The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases.[7] Methane has also been detected on other planets, including Mars, which has implications for astrobiology research.[8]
Properties and bonding
Methane is a tetrahedral molecule with four equivalent C–H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H. The lowest energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms. Above this energy level is a triply degenerate set of MOs that involve overlap of the 2p orbitals on carbon with various linear combinations of the 1s orbitals on hydrogen. The resulting "three-over-one" bonding scheme is consistent with photoelectron spectroscopic measurements.
At room temperature and standard pressure, methane is a colorless, odorless gas.[9] The familiar smell of natural gas as used in homes is achieved by the addition of an odorant, usually blends containing tert-butylthiol, as a safety measure. Methane has a boiling point of −164 °C (−257.8 °F) at a pressure of one atmosphere.[10] As a gas it is flammable over a range of concentrations (5.4–17%) in air at standard pressure.
Solid methane exists in several modifications. Presently nine are known.[11] Cooling methane at normal pressure results in the formation of methane I. This substance crystallizes in the cubic system (space group Fm3m). The positions of the hydrogen atoms are not fixed in methane I, i.e. methane molecules may rotate freely. Therefore, it is a plastic crystal.[12]
Chemical reactions
The primary chemical reactions of methane are combustion, steam reforming to syngas, and halogenation. In general, methane reactions are difficult to control.
Selective oxidation
Partial oxidation of methane to methanol is challenging because the reaction typically progresses all the way to carbon dioxide and water even with an insufficient supply of oxygen. The enzymes methane monooxygenase produces methanol from methane, but cannot be used for industrial-scale reactions.[13] Some homogeneously catalyzed systems and heterogeneous systems have been developed, but all have significant drawbacks. These generally operate by generating protected products which are shielded from overoxidation. Examples include the Catalytica system, copper zeolites, and iron zeolites stabilizing the alpha-oxygen active site.[14]
One group of bacteria drive methane oxidation with nitrite as the oxidant in the absence of oxygen, giving rise to the so-called anaerobic oxidation of methane.[15]
Acid-base reactions
Like other hydrocarbons, methane is a very weak acid. Its pKa in DMSO is estimated to be 56.[16] It cannot be deprotonated in solution, but the conjugate base is known in forms such as methyllithium.
A variety of positive ions derived from methane have been observed, mostly as unstable species in low-pressure gas mixtures. These include methenium or methyl cation CH+
3, methane cation CH+
4, and methanium or protonated methane CH+
5. Some of these have been detected in outer space. Methanium can also be produced as diluted solutions from methane with superacids. Cations with higher charge, such as CH2+
6 and CH3+
7, have been studied theoretically and conjectured to be stable.[17]
Despite the strength of its C–H bonds, there is intense interest in catalysts that facilitate C–H bond activation in methane (and other lower numbered alkanes).[18]
Combustion

Methane's heat of combustion is 55.5 MJ/kg.[19] Combustion of methane is a multiple step reaction summarized as follows:
Peters four-step chemistry is a systematically reduced four-step chemistry which explains the burning of methane.
Methane radical reactions
Given appropriate conditions, methane reacts with halogen radicals as follows:
- X• + CH4 → HX + CH3•
- CH3• + X2 → CH3X + X•
where X is a halogen: fluorine (F), chlorine (Cl), bromine (Br), or iodine (I). This mechanism for this process is called free radical halogenation. It is initiated when UV light or some other radical initiator (like peroxides) produces a halogen atom. A two-step chain reaction ensues in which the halogen atom abstracts a hydrogen atom from a methane molecule, resulting in the formation of a hydrogen halide molecule and a methyl radical (CH3•). The methyl radical then reacts with a molecule of the halogen to form a molecule of the halomethane, with a new halogen atom as byproduct.[20] Similar reactions can occur on the halogenated product, leading to replacement of additional hydrogen atoms by halogen atoms with dihalomethane, trihalomethane, and ultimately, tetrahalomethane structures, depending upon reaction conditions and the halogen-to-methane ratio.
Uses
Methane is used in industrial chemical processes and may be transported as a refrigerated liquid (liquefied natural gas, or LNG). While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas, the gas at ambient temperature is lighter than air. Gas pipelines distribute large amounts of natural gas, of which methane is the principal component.
Fuel
Methane is used as a fuel for ovens, homes, water heaters, kilns, automobiles,[21][22] turbines, and other things. Activated carbon is used to store methane. Refined liquid methane is used as a rocket fuel,[23] when combined with liquid oxygen, as in the BE-4 and Raptor engines.[24]
As the major constituent of natural gas, methane is important for electricity generation by burning it as a fuel in a gas turbine or steam generator. Compared to other hydrocarbon fuels, methane produces less carbon dioxide for each unit of heat released. At about 891 kJ/mol, methane's heat of combustion is lower than that of any other hydrocarbon. However, it produces more heat per mass (55.7 kJ/g) than any other organic molecule due to its relatively large content of hydrogen, which accounts for 55% of the heat of combustion[25] but contributes only 25% of the molecular mass of methane. In many cities, methane is piped into homes for domestic heating and cooking. In this context it is usually known as natural gas, which is considered to have an energy content of 39 megajoules per cubic meter, or 1,000 BTU per standard cubic foot. Liquefied natural gas (LNG) is predominantly methane (CH4) converted into liquid form for ease of storage or transport.
As a rocket fuel, methane offers the advantage over kerosene of depositing less carbon on the internal parts of rocket motors, reducing the difficulty of re-use of boosters.
Chemical feedstock
Natural gas, which is mostly composed of methane, is used to produce hydrogen gas on an industrial scale. Steam Methane Reforming (SMR), is the most common method of producing commercial bulk hydrogen gas. More than 50 million metric tons are produced annually worldwide (2013), principally from SMR of natural gas.[26] Much of this hydrogen is used in petroleum refineries, in the production of chemicals and in food processing. Very large quantities of hydrogen are used in the industrial synthesis of ammonia.
At high temperatures (700 – 1100 °C) and in the presence of a metal-based catalyst (nickel), steam reacts with methane to yield a mixture of CO and H2, known as "Water gas" or "Syn-gas":
This reaction is strongly endothermic (consumes heat, ΔHr= 206 kJ/mol). Additional hydrogen is obtained by the reaction of CO with water via the water-gas shift reaction.
- CO + H2O ⇌ CO2 + H2
This reaction is mildly exothermic (produces heat, ΔHr= -41 kJ/mol).
Methane is also subjected to free-radical chlorination in the production of chloromethanes, although methanol is a more typical precursor.[27]
Generation
Geological routes
The two main routes for geological methane generation are (i) organic (thermally generated, or thermogenic) and (ii) inorganic (abiotic).[8] Thermogenic methane occurs due to the breakup of organic matter at elevated temperatures and pressures in deep sedimentary strata. Most methane in sedimentary basins is thermogenic; therefore, thermogenic methane is the most important source of natural gas. Thermogenic methane components are typically considered to be relic (from an earlier time). Generally, formation of thermogenic methane (at depth) can occur through organic matter breakup, or organic synthesis. Both ways can involve microorganisms (methanogenesis), but may also occur inorganically. The processes involved can also consume methane, with and without microorganisms.
The more important source of methane at depth (crystalline bedrock) is abiotic. Abiotic means that methane is created from inorganic compounds, without biological activity, either through magmatic processes or via water-rock reactions that occur at low temperatures and pressures, like serpentinization.[28][29]
Biological routes
Most of Earth's methane is biogenic and is produced by methanogenesis,[30][31] a form of anaerobic respiration only known to be conducted by some members of the domain Archaea.[32] Methanogens occupy landfills and other soils,[33] ruminants (for example cows or cattle),[34] the guts of termites, and the anoxic sediments below the seafloor and the bottom of lakes. Rice fields also generate large amounts of methane during plant growth.[35] This multistep process is used by these microorganisms for energy. The net reaction of methanogenesis is:
- CO2 + 4 H2→ CH4 + 2 H2O
The final step in the process is catalyzed by the enzyme methyl coenzyme M reductase (MCR).[36]

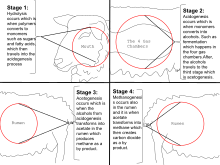
Ruminants
Ruminants, such as cattle, belch methane, accounting for ~22% of the U.S. annual methane emissions to the atmosphere.[37] One study reported that the livestock sector in general (primarily cattle, chickens, and pigs) produces 37% of all human-induced methane.[38] A 2013 study estimated that livestock accounted for 44% of human-induced methane and ~15% of human-induced greenhouse gas emissions.[39] Many efforts are underway to reduce livestock methane production, such as medical treatments and dietary adjustments,[40] and to trap the gas to use as energy.[41]
Seafloor sediments
Most of the subseafloor is anoxic because oxygen is removed by aerobic microorganisms within the first few centimeters of the sediment. Below the oxygen replete seafloor, methanogens produce methane that is either used by other organisms or becomes trapped in gas hydrates.[32] These other organisms which utilize methane for energy are known as methanotrophs (methane-eating), and are the main reason why little methane generated at depth reaches the sea surface.[32] Consortia of Archaea and Bacteria have been found to oxidize methane via Anaerobic Oxidation of Methane (AOM); the organisms responsible for this are Anaerobic Methanotrophic Archaea (ANME) and Sulfate-Reducing Bacteria (SRB).[42]
Industrial routes
There is little incentive to produce methane industrially. Methane is produced by hydrogenating carbon dioxide through the Sabatier process. Methane is also a side product of the hydrogenation of carbon monoxide in the Fischer–Tropsch process, which is practiced on a large scale to produce longer-chain molecules than methane.
Example of large-scale coal-to-methane gasification is the Great Plains Synfuels plant, started in 1984 in Beulah, North Dakota as a way to develop abundant local resources of low-grade lignite, a resource that is otherwise difficult to transport for its weight, ash content, low calorific value and propensity to spontaneous combustion during storage and transport.
Power to methane is a technology that uses electrical power to produce hydrogen from water by electrolysis and uses the Sabatier reaction to combine hydrogen with carbon dioxide to produce methane. As of 2016, this is mostly under development and not in large-scale use. Theoretically, the process could be used as a buffer for excess and off-peak power generated by highly fluctuating wind generators and solar arrays. However, as currently very large amounts of natural gas are used in power plants (e.g. CCGT) to produce electric energy, the losses in efficiency are not acceptable.
Laboratory synthesis
Methane can be produced by protonation of methyl lithium and methylmagnesium iodide. In practice, a requirement for pure methane will be filled with a steel gas bottle from standard suppliers.
Occurrence
Methane was discovered and isolated by Alessandro Volta between 1776 and 1778 when studying marsh gas from Lake Maggiore. It is the major component of natural gas, about 87% by volume. The major source of methane is extraction from geological deposits known as natural gas fields, with coal seam gas extraction becoming a major source (see Coal bed methane extraction, a method for extracting methane from a coal deposit, while enhanced coal bed methane recovery is a method of recovering methane from non-mineable coal seams). It is associated with other hydrocarbon fuels, and sometimes accompanied by helium and nitrogen. Methane is produced at shallow levels (low pressure) by anaerobic decay of organic matter and reworked methane from deep under the Earth's surface. In general, the sediments that generate natural gas are buried deeper and at higher temperatures than those that contain oil.
Methane is generally transported in bulk by pipeline in its natural gas form, or LNG carriers in its liquefied form; few countries transport it by truck.
Atmospheric methane
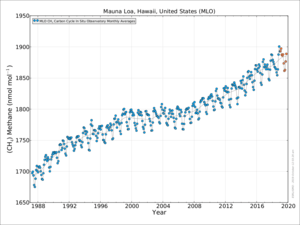
In 2010, methane levels in the Arctic were measured at 1850 nmol/mol. This level is over twice as high as at any time in the last 400,000 years. Historic methane concentrations in the world's atmosphere have ranged between 300 and 400 nmol/mol during glacial periods commonly known as ice ages, and between 600 and 700 nmol/mol during the warm interglacial periods. The Earth's oceans are a potential important source of Arctic methane.[43]
Methane is an important greenhouse gas with a global warming potential of 34 compared to CO2 (potential of 1) over a 100-year period, and 72 over a 20-year period.[44][45]
The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases (these gases don't include water vapor which is by far the largest component of the greenhouse effect).[7]
From 2015 to 2019 sharp rises in levels of atmospheric methane have been recorded.[46][47] In February 2020, it was reported methane emissions from the fossil fuel industry may have been significantly underestimated.[48]
Climate change can increase atmospheric methane levels by increasing methane production in natural ecosystems, forming a Climate change feedback.[32]
Clathrates
Methane clathrates (also known as methane hydrates) are solid cages of water molecules that trap single molecules of methane. Significant reservoirs of methane clathrates have been found in arctic permafrost and along continental margins beneath the ocean floor within the gas clathrate stability zone, located at high pressures (1 to 100 MPa; lower end requires lower temperature) and low temperatures (< 15 °C; upper end requires higher pressure).[49] Methane clathrates can form from biogenic methane, thermogenic methane, or a mix of the two. These deposits are both a potential source of methane fuel as well as a potential contributor to global warming.[50][51] The global mass of carbon stored in gas clathrates is still uncertain and has been estimated as high as 12,500 Gt carbon and as low as 500 Gt carbon.[52] The estimate has declined over time with a most recent estimate of ~1800 Gt carbon.[53] A large part of this uncertainty is due to our knowledge gap in sources and sinks of methane and the distribution of methane clathrates at the global scale. For example, a relatively newly discovered source of methane was discovered in an ultraslow spreading ridge in the Arctic.[54] Some climate models suggest that today's methane emission regime from the ocean floor is potentially similar to that during the period of the Paleocene–Eocene Thermal Maximum (PETM) around 55.5 million years ago, although there are no data indicating that methane from clathrate dissociation currently reaches the atmosphere.[53] Arctic methane release from permafrost and seafloor methane clathrates is a potential consequence and further cause of global warming; this is known as the clathrate gun hypothesis.[55][56][57][58] Data from 2016 indicate that Arctic permafrost thaws faster than predicted.[59]
Extraterrestrial methane
Interstellar medium
Methane is abundant in many parts of the Solar system and potentially could be harvested on the surface of another solar-system body (in particular, using methane production from local materials found on Mars[60] or Titan), providing fuel for a return journey.[23][61]
Mars
Methane has been detected on all planets of the solar system and most of the larger moons. With the possible exception of Mars, it is believed to have come from abiotic processes.[62][63]
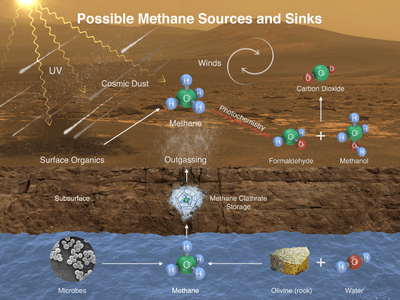
The Curiosity rover has documented seasonal fluctuations of atmospheric methane levels on Mars. These fluctuations peaked at the end of the Martian summer at 0.6 parts per billion.[64][65][66][67][68][69][70][71]
Methane has been proposed as a possible rocket propellant on future Mars missions due in part to the possibility of synthesizing it on the planet by in situ resource utilization.[72] An adaptation of the Sabatier methanation reaction may be used with a mixed catalyst bed and a reverse water-gas shift in a single reactor to produce methane from the raw materials available on Mars, utilizing water from the Martian subsoil and carbon dioxide in the Martian atmosphere.[60]
Methane could be produced by a non-biological process called serpentinization[lower-alpha 1] involving water, carbon dioxide, and the mineral olivine, which is known to be common on Mars.[73]
History
In November 1776, methane was first scientifically identified by Italian physicist Alessandro Volta in the marshes of Lake Maggiore straddling Italy and Switzerland. Volta was inspired to search for the substance after reading a paper written by Benjamin Franklin about "flammable air".[74] Volta collected the gas rising from the marsh, and by 1778 had isolated the pure gas.[75] He also demonstrated that the gas could be ignited with an electric spark.[75]
The name "methane" was coined in 1866 by the German chemist August Wilhelm von Hofmann.[76] The name was derived from methanol.
Etymology
Etymologically, the word "methane" is coined from the chemical suffix "-ane", which denotes substances belonging to the alkane family; and the word "methyl", which is derived from the German "methyl" (A.D.1840) or directly from the French "méthyle" which is a back-formation from the French "méthylène" (corresponding to English "methylene"), the root of which is coined from the Greek "methy" (related to English "mead") and "hyle" (meaning "wood"). The radical is named after this because it was first detected in wood alcohol. The chemical suffix "-ane" is from the coordinating chemical suffix "-ine" which is from Latin feminine suffix "-ina" which is applied to represent abstracts. The coordination of "-ane", "-ene", "-one", etc. was proposed in 1866 by German chemist August Wilhelm von Hofmann (1818-1892).
Safety
Methane is nontoxic, yet it is extremely flammable and may form explosive mixtures with air. Methane is also an asphyxiant if the oxygen concentration is reduced to below about 16% by displacement, as most people can tolerate a reduction from 21% to 16% without ill effects. The concentration of methane at which asphyxiation risk becomes significant is much higher than the 5–15% concentration in a flammable or explosive mixture. Methane off-gas can penetrate the interiors of buildings near landfills and expose occupants to significant levels of methane. Some buildings have specially engineered recovery systems below their basements to actively capture this gas and vent it away from the building.
Methane gas explosions are responsible for many deadly mining disasters.[77] A methane gas explosion was the cause of the Upper Big Branch coal mine disaster in West Virginia on April 5, 2010, killing 29.[78]
See also
- 2007 Zasyadko mine disaster
- Abiogenic petroleum origin
- Aerobic methane production
- Anaerobic digestion
- Anaerobic respiration
- Arctic methane emissions
- Atmospheric methane
- Biogas
- Coal Oil Point seep field
- Energy density
- Fugitive gas emissions
- Gas
- Global Methane Initiative
- Global warming
- Greenhouse gas
- Halomethane, halogenated methane derivatives.
- Hydrogen Cycle
- Industrial gas
- Lake Kivu (more general: limnic eruption)
- List of straight-chain alkanes
- Methanation
- Methane clathrate, ice that contains methane.
- Methane (data page)
- Methane emissions
- Methane on Mars: atmosphere
- Methane on Mars: climate
- Methanogen, archaea that produce methane.
- Methanogenesis, microbes that produce methane.
- Methanotroph, bacteria that grow with methane.
- Methyl group, a functional group related to methane.
- Thomas Gold
Notes
- There are many serpentinization reactions. Olivine is a solid solution between forsterite and fayalite whose general formula is (Fe,Mg)2SiO4. The reaction producing methane from olivine can be written as: Forsterite + Fayalite + Water + Carbonic acid → Serpentine + Magnetite + Methane , or (in balanced form): 18 Mg2SiO4 + 6 Fe2SiO4 + 26 H2O + CO2 → 12 Mg3Si2O5(OH)4 + 4 Fe3O4 + CH4
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 3–4. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Methane is a retained name (see P-12.3) that is preferred to the systematic name ‘carbane’, a name never recommended to replace methane, but used to derive the names ‘carbene’ and ‘carbyne’ for the radicals H2C2• and HC3•, respectively.
- "Gas Encyclopedia". Retrieved November 7, 2013.
- Pubchem. "Methane". pubchem.ncbi.nlm.nih.gov.
- "Safety Datasheet, Material Name: Methane" (PDF). USA: Metheson Tri-Gas Incorporated. December 4, 2009. Archived from the original (PDF) on June 4, 2012. Retrieved December 4, 2011.
- NOAA Office of Response and Restoration, US GOV. "METHANE". noaa.gov.
- Khalil, M. A. K. (1999). "Non-Co2 Greenhouse Gases in the Atmosphere". Annual Review of Energy and the Environment. 24: 645–661. doi:10.1146/annurev.energy.24.1.645.
- "Technical summary". Climate Change 2001. United Nations Environment Programme. Archived from the original on June 4, 2011.
- Etiope, Giuseppe; Lollar, Barbara Sherwood (2013). "Abiotic Methane on Earth". Reviews of Geophysics. 51 (2): 276–299. Bibcode:2013RvGeo..51..276E. doi:10.1002/rog.20011. ISSN 1944-9208.
- Hensher, David A. & Button, Kenneth J. (2003). Handbook of transport and the environment. Emerald Group Publishing. p. 168. ISBN 978-0-08-044103-0.
- Methane Phase change data. NIST Chemistry Webbook.
- Bini, R.; Pratesi, G. (1997). "High-pressure infrared study of solid methane: Phase diagram up to 30 GPa". Physical Review B. 55 (22): 14800–14809. Bibcode:1997PhRvB..5514800B. doi:10.1103/physrevb.55.14800.
- Wendelin Himmelheber. "Crystal structures". Retrieved December 10, 2019.
- Baik, Mu-Hyun; Newcomb, Martin; Friesner, Richard A.; Lippard, Stephen J. (2003). "Mechanistic Studies on the Hydroxylation of Methane by Methane Monooxygenase". Chemical Reviews. 103 (6): 2385–419. doi:10.1021/cr950244f. PMID 12797835.
- Snyder, Benjamin E. R.; Bols, Max L.; Schoonheydt, Robert A.; Sels, Bert F.; Solomon, Edward I. (December 19, 2017). "Iron and Copper Active Sites in Zeolites and Their Correlation to Metalloenzymes". Chemical Reviews. 118 (5): 2718–2768. doi:10.1021/acs.chemrev.7b00344. ISSN 0009-2665. PMID 29256242.
- Reimann, Joachim; Jetten, Mike S.M.; Keltjens, Jan T. (2015). "Chapter 7 Metal Enzymes in "Impossible" Microorganisms Catalyzing the Anaerobic Oxidation of Ammonium and Methane". In Peter M.H. Kroneck and Martha E. Sosa Torres (ed.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. 15. Springer. pp. 257–313. doi:10.1007/978-3-319-12415-5_7. ISBN 978-3-319-12414-8. PMID 25707470.
- Bordwell, Frederick G. (1988). "Equilibrium acidities in dimethyl sulfoxide solution". Accounts of Chemical Research. 21 (12): 456–463. doi:10.1021/ar00156a004.
- Rasul, G.; Surya Prakash, G.K.; Olah, G.A. (2011). "Comparative study of the hypercoordinate carbonium ions and their boron analogs: A challenge for spectroscopists". Chemical Physics Letters. 517 (1): 1–8. Bibcode:2011CPL...517....1R. doi:10.1016/j.cplett.2011.10.020.
- Bernskoetter, W.H.; Schauer, C.K.; Goldberg, K.I.; Brookhart, M. (2009). "Characterization of a Rhodium(I) σ-Methane Complex in Solution". Science. 326 (5952): 553–556. Bibcode:2009Sci...326..553B. doi:10.1126/science.1177485. PMID 19900892.
- Energy Content of some Combustibles (in MJ/kg) Archived January 9, 2014, at the Wayback Machine. People.hofstra.edu. Retrieved on March 30, 2014.
- March, Jerry (1968). Advance Organic Chemistry: Reactions, Mechanisms and Structure. New York: McGraw-Hill Book Company. pp. 533–534.
- "Lumber Company Locates Kilns at Landfill to Use Methane – Energy Manager Today". Energy Manager Today. Retrieved March 11, 2016.
- Cornell, Clayton B. (April 29, 2008). "Natural Gas Cars: CNG Fuel Almost Free in Some Parts of the Country". Archived from the original on January 20, 2019. Retrieved July 25, 2009.
Compressed natural gas is touted as the 'cleanest burning' alternative fuel available, since the simplicity of the methane molecule reduces tailpipe emissions of different pollutants by 35 to 97%. Not quite as dramatic is the reduction in net greenhouse-gas emissions, which is about the same as corn-grain ethanol at about a 20% reduction over gasoline
- Thunnissen, Daniel P.; Guernsey, C. S.; Baker, R. S.; Miyake, R. N. (2004). "Advanced Space Storable Propellants for Outer Planet Exploration". American Institute of Aeronautics and Astronautics (4–0799): 28.
- "Blue Origin BE-4 Engine". Retrieved June 14, 2019.
We chose LNG because it is highly efficient, low cost and widely available. Unlike kerosene, LNG can be used to self-pressurize its tank. Known as autogenous repressurization, this eliminates the need for costly and complex systems that draw on Earth’s scarce helium reserves. LNG also possesses clean combustion characteristics even at low throttle, simplifying engine reuse compared to kerosene fuels.
- Schmidt-Rohr, Klaus (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2". Journal of Chemical Education. 92 (12): 2094–2099. Bibcode:2015JChEd..92.2094S. doi:10.1021/acs.jchemed.5b00333.
- https://www.hydrogen.energy.gov/pdfs/hpep_report_2013.pdf
- Rossberg, M. et al. (2006) "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- Kietäväinen and Purkamo (2015). "The origin, source, and cycling of methane in deep crystalline rock biosphere". Front. Microbiol. 6: 725. doi:10.3389/fmicb.2015.00725. PMC 4505394. PMID 26236303.
- Cramer and Franke (2005). "Indications for an active petroleum system in the Laptev Sea, NE Siberia". Journal of Petroleum Geology. 28 (4): 369–384. Bibcode:2005JPetG..28..369C. doi:10.1111/j.1747-5457.2005.tb00088.x.
- Lessner, Daniel J(Dec 2009) Methanogenesis Biochemistry. In: eLS. John Wiley & Sons Ltd, Chichester. http://www.els.net [doi: 10.1002/9780470015902.a0000573.pub2]
- Thiel, Volker (2018), "Methane Carbon Cycling in the Past: Insights from Hydrocarbon and Lipid Biomarkers", in Wilkes, Heinz (ed.), Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate, Handbook of Hydrocarbon and Lipid Microbiology, Springer International Publishing, pp. 1–30, doi:10.1007/978-3-319-54529-5_6-1, ISBN 9783319545295
- Dean, Joshua F.; Middelburg, Jack J.; Röckmann, Thomas; Aerts, Rien; Blauw, Luke G.; Egger, Matthias; Jetten, Mike S. M.; de Jong, Anniek E. E.; Meisel, Ove H. (2018). "Methane Feedbacks to the Global Climate System in a Warmer World". Reviews of Geophysics. 56 (1): 207–250. Bibcode:2018RvGeo..56..207D. doi:10.1002/2017RG000559. hdl:1874/366386.
- Serrano-Silva, N.; Sarria-Guzman, Y.; Dendooven, L.; Luna-Guido, M. (2014). "Methanogenesis and methanotrophy in soil: a review". Pedosphere. 24: 291–307. doi:10.1016/S0009-2541(99)00092-3.
- Sirohi, S. K.; Pandey, Neha; Singh, B.; Puniya, A. K. (September 1, 2010). "Rumen methanogens: a review". Indian Journal of Microbiology. 50 (3): 253–262. doi:10.1007/s12088-010-0061-6. ISSN 0973-7715. PMC 3450062. PMID 23100838.
- IPCC. Climate Change 2013: The physical Science Basis Archived October 3, 2018, at the Wayback Machine. United Nations Environment Programme, 2013: Ch. 6, p. 507 IPCC.ch
- Lyu, Zhe; Shao, Nana; Akinyemi, Taiwo; Whitman, William B. (2018). "Methanogenesis". Current Biology. 28 (13): R727–R732. doi:10.1016/j.cub.2018.05.021. ISSN 0960-9822. PMID 29990451.
- "Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2014". 2016. Cite journal requires
|journal=(help) - FAO (2006). Livestock's Long Shadow–Environmental Issues and Options. Rome, Italy: Food and Agriculture Organization of the United Nations (FAO). Retrieved October 27, 2009.
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A. & Tempio, G. (2013). "Tackling Climate Change Through Livestock". Rome: Food and Agriculture Organization of the United Nations (FAO).
- Roach, John (May 13, 2002). "New Zealand Tries to Cap Gaseous Sheep Burps". National Geographic. Retrieved March 2, 2011.
- Silverman, Jacob (July 16, 2007). "Do cows pollute as much as cars?". HowStuffWorks.com.
- Knittel, K.; Wegener, G.; Boetius, A. (2019), McGenity, Terry J. (ed.), "Anaerobic Methane Oxidizers", Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology, Handbook of Hydrocarbon and Lipid Microbiology, Springer International Publishing, pp. 1–21, doi:10.1007/978-3-319-60063-5_7-1, ISBN 9783319600635
- "Study Finds Surprising Arctic Methane Emission Source". NASA. April 22, 2012.
- IPCC Fifth Assessment Report, Table 8.7, Chap. 8, p. 8–58 (PDF; 8,0 MB)
- Shindell, D. T.; Faluvegi, G.; Koch, D. M.; Schmidt, G. A.; Unger, N.; Bauer, S. E. (2009). "Improved Attribution of Climate Forcing to Emissions". Science. 326 (5953): 716–718. Bibcode:2009Sci...326..716S. doi:10.1126/science.1174760. PMID 19900930.
- Nisbet, E.G. (February 5, 2019). "Very Strong Atmospheric Methane Growth in the 4 Years 2014–2017: Implications for the Paris Agreement". Global Biogeochemical Cycles. 33 (3): 318–342. Bibcode:2019GBioC..33..318N. doi:10.1029/2018GB006009.
- McKie, Robin (February 2, 2017). "Sharp rise in methane levels threatens world climate targets". The Observer. ISSN 0029-7712. Retrieved July 14, 2019.
- Chelsea Harvey Methane Emissions from Oil and Gas May Be Significantly Underestimated; Estimates of methane coming from natural sources have been too high, shifting the burden to human activities E&E News via Scientific American February 21, 2020
- Bohrmann, Gerhard; Torres, Marta E. (2006), Schulz, Horst D.; Zabel, Matthias (eds.), "Gas Hydrates in Marine Sediments", Marine Geochemistry, Springer Berlin Heidelberg, pp. 481–512, doi:10.1007/3-540-32144-6_14, ISBN 9783540321446
- Miller, G. Tyler (2007). Sustaining the Earth: An Integrated Approach. U.S.A.: Thomson Advantage Books, ISBN 0534496725, p. 160.
- Dean, J. F. (2018). "Methane feedbacks to the global climate system in a warmer world". Reviews of Geophysics. 56 (1): 207–250. Bibcode:2018RvGeo..56..207D. doi:10.1002/2017RG000559. hdl:1874/366386.
- Boswell, Ray; Collett, Timothy S. (2011). "Current perspectives on gas hydrate resources". Energy Environ. Sci. 4 (4): 1206–1215. doi:10.1039/c0ee00203h. ISSN 1754-5692.
- Ruppel and Kessler (2017). "The interaction of climate change and methane hydrates". Reviews of Geophysics. 55 (1): 126–168. Bibcode:2017RvGeo..55..126R. doi:10.1002/2016RG000534.CS1 maint: uses authors parameter (link)
- "New source of methane discovered in the Arctic Ocean". phys.org. May 1, 2015. Retrieved April 10, 2019.
- "Methane Releases From Arctic Shelf May Be Much Larger and Faster Than Anticipated" (Press release). National Science Foundation (NSF). March 10, 2010.
- Connor, Steve (December 13, 2011). "Vast methane 'plumes' seen in Arctic ocean as sea ice retreats". The Independent.
- "Arctic sea ice reaches lowest extent for the year and the satellite record" (Press release). The National Snow and Ice Data Center (NSIDC). September 19, 2012.
- "Frontiers 2018/19: Emerging Issues of Environmental Concern". UN Environment. Retrieved March 6, 2019.
- Reuters (June 18, 2019). "Scientists shocked by Arctic permafrost thawing 70 years sooner than predicted". The Guardian. ISSN 0261-3077. Retrieved July 14, 2019.
- Zubrin, R. M.; Muscatello, A. C.; Berggren, M. (2013). "Integrated Mars in Situ Propellant Production System". Journal of Aerospace Engineering. 26: 43–56. doi:10.1061/(ASCE)AS.1943-5525.0000201.
- "Methane Blast". NASA. May 4, 2007. Retrieved July 7, 2012.
- Chang, Kenneth (November 2, 2012). "Hope of Methane on Mars Fades". New York Times. Retrieved November 3, 2012.
- Atreya, Sushil K.; Mahaffy, Paul R.; Wong, Ah-San (2007). "Methane and related trace species on Mars: origin, loss, implications for life, and habitability". Planetary and Space Science. 55 (3): 358–369. Bibcode:2007P&SS...55..358A. doi:10.1016/j.pss.2006.02.005.CS1 maint: uses authors parameter (link)
- Brown, Dwayne; Wendel, JoAnna; Steigerwald, Bill; Jones, Nancy; Good, Andrew (June 7, 2018). "Release 18-050 - NASA Finds Ancient Organic Material, Mysterious Methane on Mars". NASA. Retrieved June 7, 2018.
- NASA (June 7, 2018). "Ancient Organics Discovered on Mars - video (03:17)". NASA. Retrieved June 7, 2018.
- Wall, Mike (June 7, 2018). "Curiosity Rover Finds Ancient 'Building Blocks for Life' on Mars". Space.com. Retrieved June 7, 2018.
- Chang, Kenneth (June 7, 2018). "Life on Mars? Rover's Latest Discovery Puts It 'On the Table' - The identification of organic molecules in rocks on the red planet does not necessarily point to life there, past or present, but does indicate that some of the building blocks were present". The New York Times. Retrieved June 8, 2018.
- Voosen, Paul (June 7, 2018). "NASA rover hits organic pay dirt on Mars". Science. Retrieved June 7, 2018.
- ten Kate, Inge Loes (June 8, 2018). "Organic molecules on Mars". Science. 360 (6393): 1068–1069. Bibcode:2018Sci...360.1068T. doi:10.1126/science.aat2662. PMID 29880670.
- Webster, Christopher R.; et al. (June 8, 2018). "Background levels of methane in Mars' atmosphere show strong seasonal variations". Science. 360 (6393): 1093–1096. Bibcode:2018Sci...360.1093W. doi:10.1126/science.aaq0131. PMID 29880682.
- Eigenbrode, Jennifer L.; et al. (June 8, 2018). "Organic matter preserved in 3-billion-year-old mudstones at Gale crater, Mars". Science. 360 (6393): 1096–1101. Bibcode:2018Sci...360.1096E. doi:10.1126/science.aas9185. PMID 29880683.
- Richardson, Derek (September 27, 2016). "Elon Musk Shows Off Interplanetary Transport System". Spaceflight Insider. Retrieved October 3, 2016.
- Oze, C.; Sharma, M. (2005). "Have olivine, will gas: Serpentinization and the abiogenic production of methane on Mars". Geophysical Research Letters. 32 (10): L10203. Bibcode:2005GeoRL..3210203O. doi:10.1029/2005GL022691.
- Volta, Alessandro (1777) Lettere del Signor Don Alessandro Volta ... Sull' Aria Inflammable Nativa Delle Paludi [Letters of Signor Don Alessandro Volta ... on the flammable native air of the marshes], Milan, Italy: Giuseppe Marelli.
- Methane. BookRags. Retrieved January 26, 2012.
- See:
- A. W. Hofmann (1866) "On the action of trichloride of phosphorus on the salts of the aromatic monoamines," Proceedings of the Royal Society of London, 15 : 55– 62; see footnote on pp. 57–58.
- James Michael McBride (1999) "Development of systematic names for the simple alkanes". Available online at Chemistry Department, Yale University (New Haven, Connecticut). Archived March 16, 2012, at the Wayback Machine
- Dozolme, Philippe. "Common Mining Accidents". About.com.
- Lawrence Messina & Greg Bluestein (April 8, 2010). "Fed official: Still too soon for W.Va. mine rescue". News.yahoo.com. Retrieved April 8, 2010.
External links
| Wikimedia Commons has media related to Methane. |
| Look up methane in Wiktionary, the free dictionary. |
- Methane at The Periodic Table of Videos (University of Nottingham)
- International Chemical Safety Card 0291
- Gas (Methane) Hydrates -- A New Frontier – United States Geological Survey
- Lunsford, Jack H. (2000). "Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century". Catalysis Today. 63 (2–4): 165–174. doi:10.1016/S0920-5861(00)00456-9.
- CDC – Handbook for Methane Control in Mining


-Portrait-Portr_02303.tif.jpg)