Sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3, with a relatively narrow liquid range. In the gaseous form, this species is a significant pollutant, being the primary agent in acid rain.[4]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Sulfur trioxide | |||
| Systematic IUPAC name
Sulfonylideneoxidane | |||
| Other names
Sulfuric anhydride, Sulfur(VI) oxide | |||
| Identifiers | |||
3D model (JSmol) |
| ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.361 | ||
| EC Number |
| ||
| 1448 | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | UN 1829 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| SO3 | |||
| Molar mass | 80.066 g/mol | ||
| Appearance | Colorless to white crystalline solid which will fume in air.[1] Colorless liquid and gas.[2] | ||
| Odor | Varies. Vapor is pungent; like sulfur dioxide.[3] Mist is odorless.[2] | ||
| Density | 1.92 g/cm3, liquid | ||
| Melting point | 16.9 °C (62.4 °F; 290.0 K) | ||
| Boiling point | 45 °C (113 °F; 318 K) | ||
| Reacts to give sulfuric acid | |||
| Thermochemistry | |||
Std molar entropy (S |
256.77 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−395.7 kJ/mol | ||
| Hazards | |||
| Main hazards | |||
| Safety data sheet | ICSC 1202 | ||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H250, H314, H310, H300, H335 | ||
| P261, P270, P280, P305+351+338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
rat, 4 hr 375 mg/m3 | ||
| Related compounds | |||
Other cations |
Selenium trioxide Tellurium trioxide | ||
| Sulfur monoxide Sulfur dioxide | |||
Related compounds |
Sulfuric acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
It is prepared on an industrial scale as a precursor to sulfuric acid, and is also known as sulfuric anhydride.
In perfectly dry apparatus, sulfur trioxide vapor is invisible, and the liquid is transparent. However, it fumes profusely even in a relatively dry atmosphere (it has been used as a smoke agent) due to formation of a sulfuric acid mist. This vapor has no odor but is extremely corrosive.[2]
Molecular structure and bonding
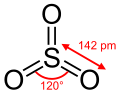
Gaseous SO3 is a trigonal planar molecule of D3h symmetry, as predicted by VSEPR theory. SO3 belongs to the D3h point group.
In terms of electron-counting formalism, the sulfur atom has an oxidation state of +6 and a formal charge of 0. The Lewis structure consists of an S=O double bond and two S–O dative bonds without utilizing d-orbitals.[5]
The electrical dipole moment of gaseous sulfur trioxide is zero. This is a consequence of the 120° angle between the S-O bonds.
Chemical reactions
SO3 is the anhydride of H2SO4. Thus, the following reaction occurs:
The reaction occurs both rapidly and exothermically, too violently to be used in large-scale manufacturing. At or above 340 °C, sulfuric acid, sulfur trioxide, and water coexist in significant equilibrium concentrations.
Sulfur trioxide also oxidizes sulfur dichloride to yield the useful reagent, thionyl chloride.
- SO3 + SCl2 → SOCl2 + SO2
SO3 is a strong Lewis acid readily forming crystalline complexes with pyridine, dioxane, and trimethylamine. These adducts can be used as sulfonating agents.[7]
Preparation
In the atmosphere
The direct oxidation of sulfur dioxide to sulfur trioxide in air:
- SO2 + ½O2 = SO3 ΔH=-198.4
does take place, but at 298 K has a ΔG = -141.6 kJ and ΔS = -187.9 J/K, so the reaction proceeds very slowly.
In the laboratory
Sulfur trioxide can be prepared in the laboratory by the two-stage pyrolysis of sodium bisulfate. Sodium pyrosulfate is an intermediate product:[8]
- Dehydration at 315 °C:
- 2 NaHSO4 → Na2S2O7 + H2O
- Cracking at 460 °C:
- Na2S2O7 → Na2SO4 + SO3
In contrast, KHSO4 does not undergo the same reaction.[8]
In industry
Industrially SO3 is made by the contact process. Sulfur dioxide, which in turn is produced by the burning of sulfur or iron pyrite (a sulfide ore of iron). After being purified by electrostatic precipitation, the SO2 is then oxidised by atmospheric oxygen at between 400 and 600 °C over a catalyst. A typical catalyst consists of vanadium pentoxide (V2O5) activated with potassium oxide K2O on kieselguhr or silica support. Platinum also works very well but is too expensive and is poisoned (rendered ineffective) much more easily by impurities.[9]
The majority of sulfur trioxide made in this way is converted into sulfuric acid not by the direct addition of water, with which it forms a fine mist, but by absorption in concentrated sulfuric acid and dilution with water of the produced oleum.
It was once produced industrially by heating calcium sulfate with silica.
Applications
Sulfur trioxide is an essential reagent in sulfonation reactions. These processes afford detergents, dyes, and pharmaceuticals. Sulfur trioxide is generated in situ from sulfuric acid or is used as a solution in the acid.
Structure of solid SO3
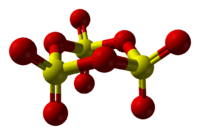
The nature of solid SO3 is complex because structural changes are caused by traces of water.[10]
Upon condensation of the gas, absolutely pure SO3 condenses into a trimer, which is often called γ-SO3. This molecular form is a colorless solid with a melting point of 16.8 °C. It adopts a cyclic structure described as [S(=O)2(μ-O)]3.[11]
If SO3 is condensed above 27 °C, then α-SO3 forms, which has a melting point of 62.3 °C. α-SO3 is fibrous in appearance. Structurally, it is the polymer [S(=O)2(μ-O)]n. Each end of the polymer is terminated with OH groups. β-SO3, like the alpha form, is fibrous but of different molecular weight, consisting of an hydroxyl-capped polymer, but melts at 32.5 °C. Both the gamma and the beta forms are metastable, eventually converting to the stable alpha form if left standing for sufficient time. This conversion is caused by traces of water.[12]
Relative vapor pressures of solid SO3 are alpha < beta < gamma at identical temperatures, indicative of their relative molecular weights. Liquid sulfur trioxide has a vapor pressure consistent with the gamma form. Thus heating a crystal of α-SO3 to its melting point results in a sudden increase in vapor pressure, which can be forceful enough to shatter a glass vessel in which it is heated. This effect is known as the "alpha explosion".[12]
SO3 is aggressively hygroscopic. The heat of hydration is sufficient that mixtures of SO3 and wood or cotton can ignite. In such cases, SO3 dehydrates these carbohydrates.[12]
Safety
Along with being a strong oxidizing agent, sulfur trioxide will cause serious burns on both inhalation and ingestion because it is highly corrosive and hygroscopic in nature. SO3 should be handled with extreme care as it reacts violently with water and produces highly corrosive sulfuric acid. It should also be kept away from organic material due to the strong dehydrating nature of sulfur trioxide and its ability to react violently with such materials.
References
- "SULFUR TRIOXIDE CAMEO Chemicals NOAA". Cameochemicals.noaa.gov.
- Lerner, L. (2011). Small-Scale Synthesis of Laboratory Reagents with Reaction Modeling. CRC Press. p. 10. ISBN 9781439813133. LCCN 2010038460.
- "Substance:Sulfur trioxide - Learn Chemistry Wiki". Rsc.org.
- Thomas Loerting; Klaus R. Liedl (2000). "Toward elimination of descrepancies between theory and experiment: The rate constant of the atmospheric conversion of SO3 to H2SO4". Proceedings of the National Academy of Sciences of the United States of America. 97 (16): 8874–8878. Bibcode:2000PNAS...97.8874L. doi:10.1073/pnas.97.16.8874. PMC 16788. PMID 10922048.
- Terence P. Cunningham , David L. Cooper , Joseph Gerratt, Peter B. Karadakov and Mario Raimondi (1997). "Chemical bonding in oxofluorides of hypercoordinate sulfur". Journal of the Chemical Society, Faraday Transactions. 93 (13): 2247–2254. doi:10.1039/A700708F.CS1 maint: multiple names: authors list (link)
- "The Manufacture of Sulfuric Acid and Superphosphate" (PDF). Chemical Processes in New Zealand.
- Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- K.J. de Vries; P.J. Gellings (May 1969). "The thermal decomposition of potassium and sodium-pyrosulfate". Journal of Inorganic and Nuclear Chemistry. 31 (5): 1307–1313. doi:10.1016/0022-1902(69)80241-1.
- Hermann Müller "Sulfuric Acid and Sulfur Trioxide" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. 2000 doi:10.1002/14356007.a25_635
- Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Merck Index of Chemicals and Drugs, 9th ed. monograph 8775