Sodium metabisulfite
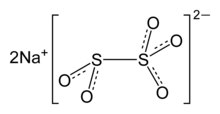
Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical formula Na2S2O5. The substance is sometimes referred to as disodium metabisulfite. It is used as a disinfectant, antioxidant, and preservative agent.[2]
 | |
 | |
| Names | |
|---|---|
| Other names
Sodium pyrosulfite Sodium disulfite | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.028.794 |
| EC Number |
|
| E number | E223 (preservatives) |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Na2S2O5, Na-O-(S=O)-O-(S=O)-O-Na | |
| Molar mass | 190.107 g/mol |
| Appearance | White to yellow powder |
| Odor | Faint SO2 |
| Density | 1.48 g/cm3 |
| Melting point | 170 °C (338 °F; 443 K) decomposition begins at 150 °C |
| |
| Solubility | Very soluble in glycerol Slightly soluble in ethanol |
| Hazards | |
| Safety data sheet | Mallinckrodt MSDS |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H318 |
| P264, P270, P280, P301+312, P305+351+338, P310, P330, P501 | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
None[1] |
REL (Recommended) |
TWA 5 mg/m3[1] |
IDLH (Immediate danger) |
N.D.[1] |
| Related compounds | |
Other anions |
Sodium sulfite Sodium bisulfite |
Other cations |
Potassium metabisulfite |
Related compounds |
Sodium dithionite Sodium thiosulfate Sodium sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Sodium disulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide.[3] When conducted in warm water, Na2SO3 initially precipitates as a yellow solid. With more SO2, the solid dissolves to give the disulfite, which crystallises upon cooling.[4]
- SO2 + 2 NaOH → Na2SO3 + H2O
- SO2 + Na2SO3 → Na2S2O5
which yields a residue of colourless solid Na2S2O5.
Chemical structure
The anion metabisulfite consists of an SO2 group linked to an SO3 group, with the negative charge more localised on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å, respectively.[5]
Reactivity
Upon dissolution in water, bisulfite is generated:
- Na2S2O5 + H2O → 2 Na+HSO3-
In this way, sodium metabisulfite is equivalent to sodium bisulfite.
Uses
Sodium and potassium metabisulfite have many major and niche uses. It is widely used for preserving food and beverages.
- Sodium metabisulphite is added as an excipient to medications which contain adrenaline (epinephrine), in order to prevent the oxidation of adrenaline.[6] For example, it is added to combination drug formulations which contain a local anaesthetic and adrenaline,[6] and to the formulation in epinephrine autoinjectors, such as the EpiPen.[7] This lengthens the shelf life of the formulation,[6] although the sodium metabisulphite reacts with adrenaline, causing it to degrade and form epinephrine sulphonate.[8]
- It is used in photography.[9]
- Concentrated sodium metabisulfite can be used to remove tree stumps. Some brands contain 98% sodium metabisulfite, and cause degradation of lignin in the stumps, facilitating removal.[10]
- It is also used as an excipient in some tablets, such as paracetamol.
- A very important health related aspect of this substance is that it can be added to a blood smear in a test for sickle cell anaemia (and other similar forms of haemoglobin mutation). The substances causes defunct cells to sickle (through a complex polymerisation) hence confirming disease.
- It is used as a bleaching agent in the production of coconut cream
- It is used as a reducing agent to break sulfide bonds in shrunken items of clothing made of natural fibres, thus allowing the garment to go back to its original shape after washing
- It is used as an SO2 source (mixed with air or oxygen) for the destruction of cyanide in commercial gold cyanidation processes.
- It is used in the water treatment industry to quench chlorine residual
- It is used in tint etching iron-based metal samples for microstructural analysis. [11] [12]
- It is used as a fungicide for anti-microbe and mould prevention during shipping of consumer goods such as shoes and clothing. Plastic stickers and packaging (such as Micro-Pak™) containing the anhydrous, sodium metabisulfite solid active ingredient are added prior to shipping. The devices absorb moisture from the atmosphere during shipping and release low levels of sulfur dioxide. [13]
- It is used for preserving fruit during shipping. [14]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0566". National Institute for Occupational Safety and Health (NIOSH).
- Barberá, José Jiménez; Metzger, Adolf; Wolf, Manfred (2000). "Sulfites, Thiosulfates, and Dithionitesl Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477.
- Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 520. ISBN 978-0-13-175553-6.
- Johnstone, H. F. (1946). "Sulfites and Pyrosulfites of the Alkali Metals". Inorganic Syntheses. 2: 162–167. doi:10.1002/9780470132333.ch49. ISBN 9780470132333.
- K. L. Carter, T. A. Siddiquee, K. L. Murphy, D. W. Bennett "The surprisingly elusive crystal structure of sodium metabisulfite" Acta Crystallogr. (2004). B60, 155–162. doi:10.1107/S0108768104003325
- McGee, Douglas L. (2013). "Local and topical anesthesia". In Roberts, James R.; Jerris, R. Hedges (eds.). Roberts and Hedges' Clinical Procedures in Emergency Medicine (6th ed.). Elsevier/Saunders. p. 519. ISBN 9781455748594.
- Niazi, Safaraz K. (2009). Handbook of Pharmaceutical Manufacturing Formulations. Volume 6, Sterile Products (2nd ed.). New York: Informa Healthcare. p. 410. ISBN 9781420081312.
- Barnes, Andrew R. (2013). "Chapter 48. Chemical stability in dosage forms". In Aulton, Michael E.; Taylor, Kevin M.G. (eds.). Aulton's pharmaceutics : the design and manufacture of medicines (4th ed.). Churchill Livingstone/Elsevier. p. 833. ISBN 9780702053931.
- Anchell, Steve (2008). The darkroom cookbook (3rd ed.). Amsterdam: Focal Press. pp. 193. ISBN 978-0240810553.
- http://www.bonideproducts.com/lbonide/msds/sds271.pdf
- "Color Metallography". 2011-05-04.
- https://www.asminternational.org/documents/10192/1874035/htp00102p025.pdf/ace8f01d-bf9a-4048-b948-a3aeb2d8a536
- "Micro-Pak Enhanced Packaging Stickers" (PDF). 2020-05-05.
- "Postharvest Biology and Technology of Tropical and Subtropical Fruits". 2020-05-05. doi:10.1533/9780857092885.361.
