Sulfur vulcanization

Sulfur vulcanization or sulfur vulcanisation is a chemical process for converting natural rubber or related polymers into more durable materials by heating them with sulphur,[1] or other equivalent curatives or accelerators. Sulfur forms cross-links (bridges) between polymer chains.[2] A vast array of products are made with vulcanized rubber, including tires, shoe soles, hoses, and conveyor belts.
The term vulcanization is derived from Vulcan, the Roman god of fire. Hard vulcanized rubber is sometimes sold under the brand name Ebonite or the genericized former brand term vulcanite, and is used in making articles such as clarinet and saxophone mouth pieces, bowling balls, and ice hockey pucks.
History
The curing of rubber has been carried out since prehistoric times.[3] The name of the first major civilization in Guatemala and Mexico, the Olmec, means 'rubber people' in the Aztec language. Ancient Mesoamericans, spanning from ancient Olmecs to Aztecs, extracted latex from Castilla elastica, a type of rubber tree in the area. The juice of a local vine, Ipomoea alba, was then mixed with this latex to create processed rubber as early as 1600 BC.[4] In the Western world, rubber remained a curiosity, although it was eventually used to produce waterproofed products, such as Mackintosh rainwear, beginning in the early 1800s.[5]
Modern developments
In 1832–1834 Nathaniel Hayward and Friedrich Ludersdorf discovered that rubber treated with sulfur lost its stickiness. It is likely Hayward shared his discovery with Charles Goodyear, possibly inspiring him to make the discovery of vulcanization.[6]
Thomas Hancock (1786–1865), a scientist and engineer, was the first to patent vulcanization of rubber. He was awarded a British patent on May 21, 1845. Three weeks later, on June 15, 1845, Charles Goodyear was awarded a patent in the United States.[7]
Goodyear claimed that he had discovered vulcanization earlier, in 1839. He wrote the story of the discovery in 1853 in his autobiographical book Gum-Elastica. Here is Goodyear's account of the invention, taken from Gum-Elastica. Although the book is an autobiography, Goodyear chose to write it in the third person so that the inventor and he referred to in the text are the author. He describes the scene in a rubber factory where his brother worked:
The inventor made experiments to ascertain the effect of heat on the same compound that had decomposed in the mail-bags and other articles. He was surprised to find that the specimen, being carelessly brought into contact with a hot stove, charred like leather.
Goodyear goes on to describe how his discovery was not readily accepted.
He directly inferred that if the process of charring could be stopped at the right point, it might divest the gum of its native adhesiveness throughout, which would make it better than the native gum. Upon further trial with heat, he was further convinced of the correctness of this inference, by finding that the India rubber could not be melted in boiling sulfur at any heat, but always charred. He made another trial of heating a similar fabric before an open fire. The same effect, that of charring the gum, followed. There were further indications of success in producing the desired result, as upon the edge of the charred portion appeared a line or border, that was not charred, but perfectly cured.
Goodyear then goes on to describe how he moved to Woburn, Massachusetts and carried out a series of systematic experiments to optimize the curing of rubber, collaborating with Nathaniel Hayward.
On ascertaining to a certainty that he had found the object of his search and much more, and that the new substance was proof against cold and the solvent of the native gum, he felt himself amply repaid for the past, and quite indifferent to the trials of the future.
Later developments
The discovery of the rubber-sulfur reaction revolutionized the use and applications of rubber, changing the face of the industrial world. Formerly, the only way to seal a small gap between moving machine parts was to use leather soaked in oil. This practice was acceptable only at moderate pressures, but above a certain point, machine designers were forced to compromise between the extra friction generated by tighter packing and greater leakage of steam. Vulcanized rubber solved this problem. It could be formed to precise shapes and dimensions, it accepted moderate to large deformations under load and recovered quickly to its original dimensions once the load is removed. These exceptional qualities, combined with good durability and lack of stickiness, were critical for an effective sealing material. Further experiments in the processing and compounding of rubber by Hancock and his colleagues led to a more reliable process.
Around 1900, disulfiram was introduced as a vulcanizing agent, and became widely used.[8]
In 1905 George Oenslager discovered that a derivative of aniline called thiocarbanilide accelerated the reaction of sulfur with rubber, leading to shorter cure times and reducing energy consumption. This breakthrough was almost as fundamental to the rubber industry as Goodyear's sulfur cure. Accelerators made the cure process faster, improved the reliability of the process and enabled vulcanization to be applied to synthetic polymers. One year after his discovery, Oenslager had found hundreds of applications for his additive. Thus, the science of accelerators and retarders was born. An accelerator speeds up the cure reaction, while a retarder delays it. A typical retarder is cyclohexylthiophthalimide. In the subsequent century chemists developed other accelerators and ultra-accelerators, that are used in the manufacture of most modern rubber goods.
Uncured vs. vulcanized rubber
In uncured rubber the long polymer chains move independently, which lets the material deform permanently (plastic behavior). Crosslinking introduced by vulcanization prevents the polymer chains from moving independently. As a result, when stress is applied the vulcanized rubber deforms, but upon release of the stress it reverts to its original shape.
Advantages of vulcanized rubber:
- Good tensile strength and extensibility
- Excellent resilience i.e. it returns to the original shape when the deforming load is removed
- Low water absorption tendency
- Higher resistance to oxidation, wear and tear, and abrasion
- Better electrical insulator
- Resistant to organic solvents (petroleum, benzene), fats and oils
- Wider useful temperature range, unlike uncured rubber which becomes soft at temperatures above 62 °C (144 °F), and brittle below 10 °C (50 °F).
Process
In contrast with thermoplastic processes (the melt-freeze process that characterize the behavior of most modern polymers), sulfur vulcanization is irreversible. It is a thermosetting polymer. The cross-linking is achieved by the addition of sulfur.
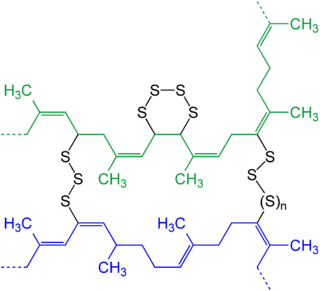
The main polymers subjected to vulcanization are polyisoprene (natural rubber) and styrene-butadiene rubber (SBR), which are used for most street-vehicle tires. The cure package is adjusted specifically for the substrate and the application. The reactive sites—cure sites—are allylic hydrogen atoms. These C-H bonds are adjacent to carbon-carbon double bonds. During vulcanization, some of these C–H bonds are replaced by chains of sulfur atoms that link with a cure site of another polymer chain. These bridges contain between one and several atoms. The number of sulfur atoms in the crosslink strongly influences the physical properties of the final rubber article. Short crosslinks give the rubber better heat resistance. Crosslinks with higher number of sulfur atoms give the rubber good dynamic properties but less heat resistance. Dynamic properties are important for flexing movements of the rubber article, e.g., the movement of a side-wall of a running tire. Without good flexing properties these movements rapidly form cracks, and ultimately make the rubber article fail.
Methods
Sulfur is a slow vulcanizing agent and does not vulcanize synthetic polyolefins. Even with natural rubber, large amounts of sulfur, as well as high temperatures and long heating periods are necessary, and one obtains an unsatisfactory crosslinking efficiency with unsatisfactory strength and aging properties. Vulcanization accelerators improve the quality of the product. Diverse additives, comprising the cure package, are necessary. The combined cure package in a typical rubber compound consists of insoluble sulfur together with compounds that modify the kinetics of crosslinking. These additives include accelerators, activators like zinc oxide, and stearic acid. The accelerators and activators are catalysts. An additional level of control is achieved by agents that inhibit vulcanization until some optimal time or temperature, which allow the uncrosslinked polymer to adapt to the mold before crosslinking. Typical retarding agents are sulfenamides. Antidegradants such as aromatic amines are added to inhibit oxidative degradation of the vulcanized product oxygen, and ozone.[9] Before vulcanization, the sulfur is not miscible in the polymer, and attention is paid to prevent sulfur bloom, where it migrates to the surface of the article.

Devulcanization
The market for new raw rubber or equivalent is large. The auto industry consumes a substantial fraction of natural and synthetic rubber. Reclaimed rubber has altered properties and is unsuitable for use in many products, including tires. Tires and other vulcanized products are potentially amenable to devulcanization, but this technology has not produced material that can supplant unvulcanized materials. The main problem is that the carbon-sulfur linkages are not readily broken, without the input of costly reagents and heat. Thus, more than half of scrap rubber is simply burned for fuel.[10]
References
- ↑ Sulfur Vulcanization of Natural Rubber for Benzothiazole Accelerated Formulations: From Reaction Mechanisms to a Rational Kinetic Model
- ↑ James E. Mark, Burak Erman (eds.) (2005). Science and technology of rubber. p. 768. ISBN 0-12-464786-3.
- ↑ Hosler, D. (18 June 1999). "Prehistoric Polymers: Rubber Processing in Ancient Mesoamerica". Science. 284 (5422): 1988–1991. doi:10.1126/science.284.5422.1988.
- ↑ D Hosler, SL Burkett and MJ Tarkanian (1999). "Prehistoric Polymers: Rubber Processing in Ancient Mesoamerica". Science. 284 (5422): 1988–1991. doi:10.1126/science.284.5422.1988. PMID 10373117.
- ↑ "Whonamedit – James Syme". Whonamedit. Retrieved 23 August 2013.
- ↑ https://books.google.com/books?id=BZtrCQAAQBAJ&pg=PP5&dq=Ludersdorf+Vulcanisation&hl=en&sa=X&ved=0ahUKEwjsm8WJnJzPAhWsK8AKHW0gDyMQ6AEIPjAG#v=onepage&q=Ludersdorf%20Vulcanisation&f=false
- ↑ 1493: Uncovering the New World Columbus Created. Random House Digital, Inc. 2011. pp. 244–245. ISBN 9780307265722.
- ↑ Kragh, Helge (2008). "From Disulfiram to Antabuse: The Invention of a Drug" (PDF). Bulletin for the History of Chemistry. 33 (2): 82–88.
- 1 2 Hans-Wilhelm Engels, Herrmann-Josef Weidenhaupt, Manfred Pieroth, Werner Hofmann, Karl-Hans Menting, Thomas Mergenhagen, Ralf Schmoll, Stefan Uhrlandt "Rubber, 4. Chemicals and Additives" in Ullmann's Encyclopedia of Industrial Chemistry 2004, Wiley-VCH, Weinheim. doi:10.1002/14356007.a23_365.pub2
- ↑ Myhre, Marvin; MacKillop, Duncan A. "Rubber Recycling" Rubber Chemistry and Technology (2002), volume 75, number 3, pp 429–474. doi:10.5254/1.3547678