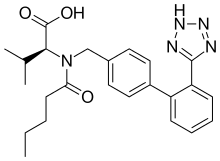
Valsartan
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Diovan (Novartis) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697015 |
| License data | |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25% |
| Protein binding | 95% |
| Elimination half-life | 6 hours |
| Excretion | Renal 30%, biliary 70% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.113.097 |
| Chemical and physical data | |
| Formula | C24H29N5O3 |
| Molar mass | 435.519 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Valsartan (trade name Diovan, Novartis International AG) is mainly used for treatment of high blood pressure, congestive heart failure, and to increase the chances of living longer after a heart attack. It is an angiotensin II receptor antagonist (commonly called an ARB, or angiotensin receptor blocker), that is selective for the type I (AT1) angiotensin receptor.
Medical uses
Valsartan is used to treat high blood pressure, congestive heart failure, and to reduce death for people with left ventricular dysfunction after having had a heart attack.[1][2]
There is contradictory evidence with regard to treating people with heart failure with a combination of an angiotensin receptor blocker like valsartan and an angiotensin-converting enzyme inhibitor, with two major clinical trials (CHARM-additive and ValHeFt) showing a reduction in death, and two others (VALIANT and ONTARGET) showing no benefits, and more adverse effects including heart attacks.[1]
In people with type II diabetes and high blood pressure or albumin in the urine, valsartan is used to slow the worsening and the development of end-stage kidney disease.[3]
Contraindications
The packaging for valsartan includes a warning stating the drug should not be used with the renin inhibitor aliskiren in people with diabetes mellitus. It also states the drug should not be used in people with kidney disease.[2]
Valsartan falls in FDA pregnancy category D and includes a black box warning for fetal toxicity.[2] Discontinuation of these agents is recommended immediately after detection of pregnancy and an alternative medication should be started.[2] The US labeling makes no recommendation regarding continuation or discontinuation of valsartan for breast-feeding mothers.[2] The Canadian labeling does not recommend use by nursing women.[4]
Side effects
Rates of side effects depends on the reason the medication is used.
Heart failure
Rates of adverse effects are based on a comparison versus placebo in people with heart failure.[5] Most common side effects include dizziness (17% vs 9% ), low blood pressure (7% vs 2%), and diarrhea (5% vs 4%).[5] Less common side effects include joint pain, fatigue, and back pain (all 3% vs 2%).[5]
Hypertension
Clinical trials for valsartan treatment for hypertension versus placebo demonstrate side effects like viral infection (3% vs 2%), fatigue (2% vs 1%) and abdominal pain (2% vs 1%). Minor side effects that occurred at >1% but were similar to rates from the placebo group include:[5]
- headache
- dizziness
- upper respiratory infection
- cough
- diarrhea
- rhinitis/sinusitis
- nausea
- pharyngitis
- edema
- arthralgia
Interactions
%2C_Singapore_-_20150210.jpg)
The US prescribing information lists the following drug interactions for valsartan:
- Other inhibitors of the renin-angiotensin system may increase the risks of low blood pressure, kidney problems, and hyperkalemia
- Potassium sparing diuretics, potassium supplements, salt substitutes containing potassium may increase the risk of hyperkalemia.
- NSAIDs may increase the risk of kidney problems and may interfere with blood pressure-lowering effects.
- Valsartan may increase the concentration of lithium.[2]
- Valsartan and other angiotensin-related blood pressure medications may interact with the antibiotics co-trimoxazole or ciprofloxacin to increase risk of sudden death due to cardiac arrest.[6]
Mechanism of action
Valsartan blocks the actions of angiotensin II, which include constricting blood vessels and activating aldosterone, to reduce blood pressure.[7] The drug binds to angiotensin type I receptors (AT1), working as an antagonist. This mechanism of action is different than that of the ACE inhibitor drugs, which block the conversion of angiotensin I to angiotensin II. As valsartan acts at the receptor, it can provide more complete angiotensin II antagonism since angiotensin II is generated by other enzymes as well as ACE. Also, valsartan does not affect the metabolism of bradykinin like ACE inhibitors do.[7]
Economics
In 2010, valsartan (trade name Diovan) achieved annual sales of $2.052 billion in the United States and $6.053 billion worldwide.[8] The patents for valsartan and valsartan/hydrochlorothiazide expired in September 2012.[9][10]
Research
In people with impaired glucose tolerance, valsartan may decrease the incidence of developing diabetes mellitus type 2. However, the absolute risk reduction is small (less than 1 percent per year) and diet, exercise or other drugs, may be more protective. In the same study, no reduction in the rate of cardiovascular events (including death) was shown.[11]
In one study of people without diabetes, valsartan reduced the risk of developing diabetes mellitus over amlodipine, mainly for those with hypertension.[12]
A prospective study demonstrated a reduction in the incidence and progression of Alzheimer's disease and dementia.[13]
Combination drugs
Valsartan is combined with amlodipine or HCTZ (or both) into single-pill formulations for treating hypertension with multiple drugs.
Recall of some generic versions
On July 6, 2018, the European Medicines Agency recalled certain batches of valsartan and valsartan/HCT film-coated tablets distributed in 22 countries in Europe, plus Canada.[14] Zhejiang Huahai Pharmaceutical Co. (ZHP) in Linhai, China manufactured pills contaminated by N-nitrosodimethylamine (NDMA), a carcinogen. This product was subsequently imported by Novartis and marketed in Europe and Asia under their subsidiary Sandoz labeling, and in the UK by Dexcel Pharma Ltd and Accord Healthcare.[14] In Canada, the recall involves five companies and a class action suit has been initiated by a private law firm.[15][16] Authorities believe the degree of contamination is negligible, and advise those taking the drug to consult a doctor and not to cease taking the medication abruptly. On July 12, 2018, The National Agency of Drug and Food Control (NA-DFC or Badan POM Indonesia) announced voluntary recalls for two products containing valsartan produced by Actavis Indonesia and Dipa Pharmalab Intersains.[17] On July 13, 2018, the FDA announced voluntary recalls of certain supplies of valsartan and valsartan/hydrochlorothiazide (HCTZ) in the U.S. distributed by Solco Healthcare LLC, Major Pharmaceuticals, and Teva Pharmaceutical Industries.[18] Hong Kong's Department of Health initiated a similar recall.[19] On August 2, 2018, the FDA published two lengthy, updated lists, classifying hundreds of specific U.S. products containing valsartan into those included versus excluded from the recall.[20] A week later, the FDA cited two more drugmakers, Zhejiang Tianyu Pharmaceuticals of China and Hetero Labs Limited of India, as additional sources of the contaminated valsartan ingredient.[21]
In September the FDA announced that retesting of all valsartan supplies had found a second carcinogenic impurity, N-nitrosodiethylamine (NDEA), in the recalled products made by ZHP in China and marketed in the U.S. under the Torrent Pharmaceuticals (India) brand.[22]
See also
References
- 1 2 Randa, Hilal-Dandan (2011). "Chapter 26. Renin and Angiotensin". In Brunton, L. L.; Chabner, Bruce; Knollmann, Björn C. Goodman & Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 978-0-07-162442-8.
- 1 2 3 4 5 6 "Diovan prescribing information" (PDF). Novartis.
- ↑ Inzucchi, Silvio E.; Bergenstal, Richard M.; Buse, John B.; Diamant, Michaela; Ferrannini, Ele; Nauck, Michael; Peters, Anne L.; Tsapas, Apostolos; Wender, Richard (2015-01-01). "Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes". Diabetes Care. 38 (1): 140–149. doi:10.2337/dc14-2441. ISSN 0149-5992. PMID 25538310.
- ↑ "DIOVAN Product Monograph". Health Canada Drug Product Database. Novartis Pharmaceuticals Canada Inc. Retrieved 5 November 2015.
- 1 2 3 4 "DailyMed - VALSARTAN - valsartan tablet". dailymed.nlm.nih.gov. Retrieved 2015-11-04.
- ↑ name="BMJ" https://doi.org/10.1136/bmj.g6196
- 1 2 Katzung, Bertram G; Trevor, Anthony J. (2015). "Chapter 11". Basic & Clinical Pharmacology (13 ed.). McGraw-Hill Education. ISBN 978-0071825054.
- ↑ "Novartis Annual Report 2010" (PDF).
- ↑ Philip Moeller (April 29, 2011). "Blockbuster Drugs That Will Go Generic Soon". U.S.News & World Report.
- ↑ Eva Von Schaper (August 5, 2011). "Novartis's Jimenez Has Blockbuster Plans For Diovan After Patent Expires". Bloomberg.
- ↑ McMurray JJ, Holman RR, Haffner SM, et al. (April 2010). "Effect of valsartan on the incidence of diabetes and cardiovascular events" (PDF). The New England Journal of Medicine. 362 (16): 1477–90. doi:10.1056/NEJMoa1001121. PMID 20228403.
- ↑ Kjeldsen SE1, McInnes GT, Mancia G, Hua TA, Julius S, Weber MA, Coca A, Girerd X, Jamerson K, Larochelle P, Macdonald T, Schmieder RE, Anthony Schork M, Viskoper R, Widimsky J, Zanchetti A. "Progressive effects of valsartan compared with amlodipine in prevention of diabetes according to categories of diabetogenic risk in hypertensive patients: the VALUE trial". Blood Press. 17: 170–7. doi:10.1080/08037050802169644. PMID 18608200.
- ↑ Li NC, Lee A, Whitmer RA, et al. (January 2010). "Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis" (PDF). BMJ. 340: b5465. doi:10.1136/bmj.b5465. PMC 2806632. PMID 20068258.
- 1 2 Christensen, Jen. "Common heart drug recalled in 22 countries for possible cancer link". CNN. Cable News Network. Retrieved 14 July 2018.
- ↑ Canada, Government of Canada, Health. "Several drugs containing valsartan being recalled due to contamination with a potential carcinogen". healthycanadians.gc.ca. Retrieved 2018-07-15.
- ↑ "Valsartan Class Action". valsartanclassaction.com. Retrieved 2018-07-15.
- ↑ RI, Badan POM. "Penjelasan BPOM RI tentang penarikan obat antihipertensi yang mengandung zat aktif valsartan". pom.go.id (in Indonesian). Retrieved 2018-07-18.
- ↑ CNN, Jen Christensen,. "FDA joins 22 countries' recall of common heart drug". CNN. Retrieved 2018-07-15.
- ↑ "Hong Kong health department issues recall for five heart drugs containing valsartan that was made in China". South China Morning Post. July 20, 2018. Retrieved July 20, 2018.
- ↑ "FDA updates on valsartan recalls". U.S. FDA Drug Safety and Availability. August 2, 2018. Retrieved August 8, 2018.
- ↑ Patrice Wendling (August 13, 2018). "More Drug Makers Tagged as Valsartan Recall Grows". WebMD. Retrieved August 13, 2018.
- ↑ "FDA provides update on its ongoing investigation into valsartan products; and reports on the finding of an additional impurity identified in one firm's already recalled products". U.S. FDA News and Events. September 13, 2018. Retrieved September 14, 2018.
External links
- Valsartan National Library of Medicine: Drug Information Portal