Tripotassium phosphate
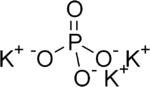 | |
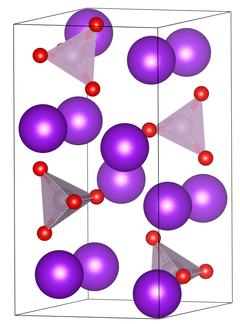 Unit cell of tripotassium phosphate. | |
| Names | |
|---|---|
| IUPAC name
Potassium phosphate | |
| Systematic IUPAC name
Potassium tetraoxidophosphate(3−) | |
| Other names
Potassium phosphate, tribasic | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.006 |
PubChem CID |
|
| |
| |
| Properties | |
| K3PO4 | |
| Molar mass | 212.27 g/mol |
| Appearance | White deliquescent powder |
| Density | 2.564 g/cm3 (17 °C) |
| Melting point | 1,380 °C (2,520 °F; 1,650 K) |
| 90 g/100 mL (20 °C) | |
| Solubility in ethanol | Insoluble |
| Basicity (pKb) | 1.6 |
| Structure[1] | |
| Primitive orthorhombic | |
| Pnma, No. 62 | |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | MSDS |
| R-phrases (outdated) | R36-R38 |
| S-phrases (outdated) | S26-S36 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
Other cations |
Trisodium phosphate Triammonium phosphate Tricalcium phosphate |
Related compounds |
Monopotassium phosphate Dipotassium phosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tripotassium phosphate, also called potassium phosphate tribasic,[3] is a water-soluble ionic salt which has the chemical formula K3PO4. It has a molecular weight of 212.27 g/mol in its anhydrous form. The compound is slightly hygroscopic. However, it has a very high melting point of 1380 °C which allows it to be heated to remove water without decomposing the salt.[4] Tripotassium phosphate is a strong inorganic base and has been used as a catalyst for many reactions. It is a tribasic salt which can be used as a food additive or to form buffer solutions in water. Besides its use as a catalyst, it has also been used for its antimicrobial properties. It is used as a food additive for its properties as an emulsifier, foaming agent, and whipping agent.[5] In combination with fatty acids, it is a potential antimicrobial agent in poultry processing.[6] It is relatively inexpensive compound[7] compared to other chlorine compounds like calcium hypochlorite (Ca(OCl)2)[8] and sodium hypochlorite solution (NaOCl)[9] which are used for killing bacteria in water. As a fertilizer, its proportions of N, P2O5, and K2O are 0-33-67.
Properties
Tripotassium phosphate can be colorless to white and be found as either crystals, lumps or powder form.[10] It has a high melting point of 1380 °C.[11] It is slightly hygroscopic. Tripotassium phosphate is a strong inorganic base with a pH of 11.5-12.5.[12]

Production
Tripotassium phosphate can be produced by the reaction of ammonium phosphate ( ) with potassium chloride ( ).[13]
Uses
Catalysis
Tripotassium phosphate has been used a catalyst for many organic reactions. It is cost-effective and has been used as an efficient catalyst to replace more expensive alternatives. Some of the reactions catalysed by are listed below:
- Hydrated tripotassium phosphate ( ) can be used as a catalyst for the removal of BOC protecting group from secondary BOC amines using methanol as a solvent. Microwave radiation is used to aid the reaction.[14]
- is used as a catalyst for the synthesis of unsymmetrical diaryl ethers using [Bmim]BF4 as the solvent. Aryl methane-sulfonates are deprotected and then followed by a nucleophilic aromatic substitution (SNAr) with activated aryl halides.[15]
- was found to be one of the catalysts that aids in the coupling reaction of aryl halides with terminal alkynes. It also plays a role in the deacetonation of 4-aryl-2-methylbut-3-yn-2-ol intermediates.[16]
- can be used as one of the catalysts for the addition of aryl halides to phenols and aliphatic alcohols.[17]
Microbicidal Use for Poultry Farming
In poultry farming, psychotrophic bacteria (extremophiles surviving in cold temperatures) pose threats to fresh refrigerated poultry. Tripotassium phosphate has been used as an alternative to chlorine to in treating poultry.
A study conducted with tripotassium phosphate showed that the compound possesses antimicrobial activities and significantly reduced bacteria on poultry skin when treated with a tripotassium phosphate solution. The tripotassium phosphate solutions were 2-4 % (wt/vol).
No viable cells were recovered when gram-negative bacteria were suspended in tripotassium phosphate solutions 2-4 % (wt/vol). However, viable cells of gram-positive bacteria (L. monocytogenes and S. aureus) and yeasts (C. ernobii and Y. lipolytica) were recovered following suspension in tripotassium phosphate solutions under the same conditions. Other studies had similar findings suggesting that gram-negative bacteria are more susceptible to the antibactericidal activity of tripotassium phosphate than gram-positive bacteria. A possible explanation for the findings is that the cell structure of gram-positive bacteria and yeasts confer them greater protection from the effect of tripotassium phosphate.
Tripotassium phosphate and fatty acids (lauric myristic acids) were mixed and also used to treat poultry skin. These solutions eliminated more gram-positive bacteria than tripotassium phosphate by itself. The antibacterial activity of fatty acids is related to their ability to reduce the surface tension (surfactants) of the cell walls of mircoorganisms and make them susceptible to external factors that can kill them.
Another study showed that the combination of tripotassium phosphate and potassium oleate significantly reduces the number of aerobic bacteria, Enterobacteriaceae, Campylobacter, and enterococci recovered from poultry skin.
Hazards
Tripotassium phosphate can cause severe eye and skin irritations. It could also cause irritations of the respiratory tract.[18]
References
- ↑ doi:10.1134/S0020168506080206
- ↑ doi:10.1134/S0020168506080206
- ↑ "Potassium phosphate tribasic P5629". Sigma-Aldrich. Retrieved 2018-04-27.
- ↑ Beitia, Johant (2010-12-14). "Tripotassium Phosphate: From Buffers to Organic Synthesis". Synlett. 2011 (01): 139–140. doi:10.1055/s-0030-1259092. ISSN 0936-5214.
- ↑ "NutritionData Food Additive Identifier". NutritionData.com.
- ↑ Hinton, Arthur; Ingram, Kimberly D. (July 2005). "Microbicidal Activity of Tripotassium Phosphate and Fatty Acids toward Spoilage and Pathogenic Bacteria Associated with Poultry". Journal of Food Protection (7): 1336–1534.
- ↑ "Potassium phosphate tribasic P5629". Sigma-Aldrich. Retrieved 2018-04-27.
- ↑ "Calcium hypochlorite 211389". Ca(OCl)2. Retrieved 2018-04-27.
- ↑ "Sodium hypochlorite solution | Sigma-Aldrich". www.sigmaaldrich.com. Retrieved 2018-04-27.
- ↑ http://www.ffcr.or.jp/zaidan/ffcrhome.nsf/7bd44c20b0dc562649256502001b65e9/916cae3da5a8a11b49256f320018877f/$FILE/D400.pdf
- ↑ Beitia, Johant (2010-12-14). "Tripotassium Phosphate: From Buffers to Organic Synthesis". Synlett. 2011 (01): 139–140. doi:10.1055/s-0030-1259092. ISSN 0936-5214.
- ↑ http://www.ffcr.or.jp/zaidan/ffcrhome.nsf/7bd44c20b0dc562649256502001b65e9/916cae3da5a8a11b49256f320018877f/$FILE/D400.pdf
- ↑ Cyclic process for producing tripotassium phosphate and ammonium chloride, 1968-10-15, retrieved 2018-04-27
- ↑ "Microwave-assisted N-Boc deprotection under mild basic conditions using K3PO4·H2O in MeOH". Tetrahedron Letters. 50 (9): 1071–1074. 2009-03-04. doi:10.1016/j.tetlet.2008.12.074. ISSN 0040-4039.
- ↑ Xu, Hui; Chen, Yang (2007-04-30). "C(aryl)-O Bond Formation from Aryl Methanesulfonates via Consecutive Deprotection and SNAr Reactions with Aryl Halides in an Ionic Liquid". Molecules. 12 (4): 861–867. doi:10.3390/12040861.
- ↑ "A simple catalyst system for the palladium-catalyzed coupling of aryl halides with terminal alkynes". Tetrahedron. 61 (41): 9878–9885. 2005-10-10. doi:10.1016/j.tet.2005.07.099. ISSN 0040-4020.
- ↑ Niu, Jiajia; Zhou, Hua; Li, Zhigang; Xu, Jingwei; Hu, Shaojing (2008-10-03). "An Efficient Ullmann-Type C−O Bond Formation Catalyzed by an Air-Stable Copper(I)−Bipyridyl Complex". The Journal of Organic Chemistry. 73 (19): 7814–7817. doi:10.1021/jo801002c. ISSN 0022-3263.
- ↑ Pubchem. "Tripotassium phosphate". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-04-27.
