Potassium metabisulfite
 | |
 | |
| Names | |
|---|---|
| Other names
Potassium pyrosulfite Dipotassium disulfite Potassium metabisulphite Dipotassium disulphite | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.037.072 |
| E number | E224 (preservatives) |
PubChem CID |
|
| RTECS number | TT4920000 |
| UNII | |
| |
| |
| Properties | |
| K2O5S2 | |
| Molar mass | 222.31 g·mol−1 |
| Appearance | White crystalline powder |
| Odor | pungent (sulfur dioxide) |
| Density | 2.34 g/cm3 (solid) |
| Melting point | 190 °C (374 °F; 463 K) decomposes |
| 450 g/l (20 °C) | |
| Solubility | insoluble in ethanol |
| Hazards | |
| Main hazards | Irritant, asthma risk |
| Safety data sheet | ICSC 1175 |
| GHS pictograms | 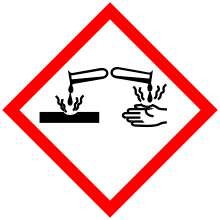  |
| GHS signal word | Danger |
| H315, H318, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P310, P312, P321, P332+313, P362, P403+233, P405, P501 | |
| NFPA 704 | |
| Related compounds | |
Other anions |
Potassium bisulfite Potassium sulfite |
Other cations |
Sodium metabisulfite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium metabisulfite, K2S2O5, also known as potassium pyrosulfite, is a white crystalline powder with a pungent sulfur odour. The main use for the chemical is as an antioxidant or chemical sterilant. It is a disulfite and is chemically very similar to sodium metabisulfite, with which it is sometimes used interchangeably. Potassium metabisulfite is generally preferred out of the two as it does not contribute sodium to the diet.
Potassium metabisulfite has a monoclinic crystal structure which decomposes at 190 °C, yielding potassium sulfite and sulfur dioxide:
- K2S2O5(s) → K2SO3(s) + SO2(g)
Uses
It is used as a food additive, also known as E224.[1] It is restricted in use and may cause allergic reactions in some sensitive persons.[2]
Potassium metabisulfite is an inhibitor of the polyphenol oxidase enzyme.[3]
Wine
Potassium metabisulfite is a common wine or must additive, in which it forms sulfur dioxide gas (SO2). This both prevents most wild microorganisms from growing, and it acts as a potent antioxidant, protecting both the color and delicate flavors of wine.
The typical dosage is 1/4 tsp (1.23 milliliters) potassium metabisulfite per six-gallon bucket of must (yielding roughly 75 ppm of SO2) prior to fermentation; then 1/2 tsp per six-gallon bucket (150 ppm of SO2) at bottling. Most commercial wineries do not add more than 30ppm at bottling.
Winemaking equipment is sanitized by spraying with a 1% SO2 (2 tsp potassium metabisulfite per L) solution.
Beer
Potassium metabisulfite is sometimes used in the brewing industry to inhibit the growth of wild bacteria and fungi. This is called 'stabilizing'. It is also used to neutralize chloramine that has been added to tap water at the source as a disinfectant. It is used both by homebrewers and commercial brewers alike. It is not used as much for brewing beer, because the wort is almost always boiled, which kills most microorganisms. It can also be added to strike water (the water used to mash the malted barley) in order to remove chloramines which can cause phenolic off flavors in beer.
Other uses
- Potassium metabisulfite is sometimes added to lemon juice as a preservative.
- Potassium metabisulfite is used in the textile industry for dyeing and cotton printing.
- Potassium metabisulfite is sometimes used to precipitate gold from solution in aqua regia (as an alternative to sodium sulfite).
- It is a component of certain photographic developers and solutions used in photographic fixing.[4]
- It is used as a bleaching agent in the production of Coconut cream.
- It is used in some pickles as a preservative.
- It is used in tint etching iron-based metal samples for microstructural analysis. [5]
Safety
Potassium metabisulfite causes skin irritation, serious eye irritation, and may cause respiratory irritation.[6] Hence, it should be manipulated under individual protective elements, such as gloves, coat, mask and glasses. Also, it should be manipulated under alkaline conditions as potassium metabisulfite reacts with acids, liberating toxic gases.
See also
References
- ↑ List of E-number food additives
- ↑ Metcalfe, Dean D.; Simon, Ronald A. (2003). Food allergy: adverse reactions to food and food additives. Malden, MA: Wiley-Blackwell. pp. 324–339. ISBN 0-632-04601-5.
- ↑ Del Signore A, Romeoa F, Giaccio M (May 1997). "Content of phenolic substances in basidiomycetes". Mycological Research. 101 (5): 552–556. doi:10.1017/S0953756296003206.
- ↑ http://encyclopedia2.thefreedictionary.com/potassium+metabisulfite
- ↑ https://vacaero.com/information-resources/metallography-with-george-vander-voort/991-color-metallography.html
- ↑ "Material Safety Data Sheet". Guidechem.
