Triose
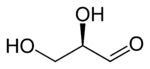
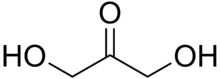
Dihydroxyacetone is a ketotriose because the carbonyl group is the center of the chain.
A triose is a monosaccharide, or simple sugar, containing three carbon atoms. There are only three possible trioses (including Dihydroxyacetone): L-Glyceraldehyde and D-Glyceraldehyde, the two enantiomers of glyceraldehyde, which are aldotrioses because the carbonyl group is at the end of the chain, and dihydroxyacetone, the only ketotriose, which is symmetrical and therefore has no enantiomers.[1]
Trioses are important in cellular respiration. During glycolysis, fructose-1,6-bisphosphate is broken down into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. Lactic acid and pyruvic acid are later derived from these molecules.[2]
References
- ↑ "Trioses - Three Carbon Sugars". Oxford University Press. Retrieved 2011-07-10.
- ↑ "Glycolysis in Detail". Ohio State University at Mansfield. Retrieved 2011-07-10.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.