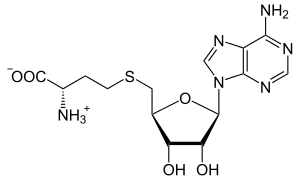
S-Adenosyl-L-homocysteine
 | |
| Names | |
|---|---|
| IUPAC name
S-(5'-Deoxyadenos-5'-yl)-L-homocysteine | |
| Other names
AdoHcy, 2-S-adenosyl-L-homocysteine, 5'-S-(3-Amino-3-carboxypropyl)-5'-thioadenosine S-adenosylhomocysteine, SAH | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.328 |
| KEGG | |
| MeSH | S-Adenosylhomocysteine |
PubChem CID |
|
| |
| |
| Properties | |
| C14H20N6O5S | |
| Molar mass | 384.412 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
S-Adenosyl-L-homocysteine (SAH) is an amino acid derivative used in several metabolic pathways in most organisms. It is an intermediate in the synthesis of cysteine and adenosine.[1]
SAH is formed by the demethylation of S-adenosyl-L-methionine and is converted to homocysteine and adenosine by adenosylhomocysteinase.
References
- ↑ James, S. Jill; Melnyk, Stepan; Pogribna, Marta; Pogribny, Igor P; Caudill, Marie A (2002). "Elevation in S-Adenosylhomocysteine and DNA Hypomethylation: Potential Epigenetic Mechanism for Homocysteine-Related Pathology". The Journal of Nutrition. 132 (8): 2361S–2366S. doi:10.1093/jn/132.8.2361S.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.