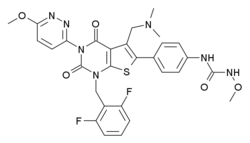
Relugolix
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | RVT-601; TAK-385 |
| Routes of administration | By mouth |
| Drug class | GnRH antagonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C29H27F2N7O5S |
| Molar mass | 623.630 g/mol |
| 3D model (JSmol) | |
| |
| |
Relugolix (developmental code names RVT-601, TAK-385) is a gonadotropin-releasing hormone antagonist (GnRH antagonist) medication which is under development by Myovant Sciences and Takeda for the treatment of endometriosis, uterine fibroids, and prostate cancer.[1][2][3][4][5][6][7] Unlike most other GnRH modulators, but similarly to elagolix, relugolix is a non-peptide and small-molecule compound and is orally active.[6][7] As of July 2018, it is in the pre-registration phase of development for uterine fibroids and is in phase III clinical trials for endometriosis and prostate cancer.[1]
Pharmacology
Pharmacodynamics
Relugolix is a selective antagonist of the gonadotropin-releasing hormone receptor (GnRHR) (IC50 = 0.12 nM).[6][7][8]
A single oral administration of relugolix at a dose of 3 mg/kg has been found to suppress luteinizing hormone (LH) levels for more than 24 hours in castrated cynomolgus monkeys, indicating a long duration of action.[6] The drug (80–160 mg/day) has been found to reduce testosterone levels to sustained castrate levels in men with once-daily administration.[8] Lower dosages (10–40 mg/day) are being studied in the treatment of endometriosis and uterine fibroids to achieve partial sex hormone suppression.[4] The reasoning behind partial suppression for these conditions is to reduce the incidence and severity of menopausal symptoms such as hot flushes and to avoid bone mineral density changes caused by estrogen deficiency that can eventually lead to osteoporosis.[4][9]
History
Relugolix was first described in 2004.[10][6] It superseded sufugolix, which was developed by the same group.[6]
Society and culture
Generic names
Relugolix is the generic name of the drug and its INN and USAN.[11] It is also known by its developmental code names RVT-601 and TAK-385.[1][11]
See also
References
- 1 2 3 http://adisinsight.springer.com/drugs/800028257
- ↑ Goenka L, George M, Sen M (June 2017). "A peek into the drug development scenario of endometriosis - A systematic review". Biomed. Pharmacother. 90: 575–585. doi:10.1016/j.biopha.2017.03.092. PMID 28407578.
- ↑ Dellis A, Papatsoris A (October 2017). "Therapeutic outcomes of the LHRH antagonists". Expert Rev Pharmacoecon Outcomes Res. 17 (5): 481–488. doi:10.1080/14737167.2017.1375855. PMID 28870102.
- 1 2 3 Streuli I, de Ziegler D, Borghese B, Santulli P, Batteux F, Chapron C (March 2012). "New treatment strategies and emerging drugs in endometriosis". Expert Opin Emerg Drugs. doi:10.1517/14728214.2012.668885. PMID 22439891.
- ↑ Elancheran, R.; Maruthanila, V. L.; Ramanathan, M.; Kabilan, S.; Devi, R.; Kunnumakara, A.; Kotoky, Jibon (2015). "Recent discoveries and developments of androgen receptor based therapy for prostate cancer". Med. Chem. Commun. 6 (5): 746–768. doi:10.1039/C4MD00416G. ISSN 2040-2503.
- 1 2 3 4 5 6 Miwa K, Hitaka T, Imada T, Sasaki S, Yoshimatsu M, Kusaka M, Tanaka A, Nakata D, Furuya S, Endo S, Hamamura K, Kitazaki T (July 2011). "Discovery of 1-{4-[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gonadotropin-releasing hormone receptor". J. Med. Chem. 54 (14): 4998–5012. doi:10.1021/jm200216q. PMID 21657270.
- 1 2 3 Nakata D, Masaki T, Tanaka A, Yoshimatsu M, Akinaga Y, Asada M, Sasada R, Takeyama M, Miwa K, Watanabe T, Kusaka M (January 2014). "Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: studies in human GnRH receptor knock-in mice". Eur. J. Pharmacol. 723: 167–74. doi:10.1016/j.ejphar.2013.12.001. PMID 24333551.
- 1 2 MacLean D, Shi H, Suri A, Faessel H, and Saad F (2013). "Safety and Testosterone-Lowering Effects of the Investigational, Oral, GnRH Antagonist, TAK-385 in Healthy Male Volunteers: Results of a Phase 1 Inpatient/Outpatient Study". doi:10.1210/endo-meetings.2013.CN.1.SAT-318.
- ↑ Struthers RS, Nicholls AJ, Grundy J, Chen T, Jimenez R, Yen SS, Bozigian HP (February 2009). "Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix". J. Clin. Endocrinol. Metab. 94 (2): 545–51. doi:10.1210/jc.2008-1695. PMC 2646513. PMID 19033369.
- ↑ https://patents.google.com/patent/US7300935/
- 1 2 https://chem.nlm.nih.gov/chemidplus/rn/737789-87-6
External links