Pollen tube

A pollen tube is a tubular structure produced by the male gametophyte of seed plants when it germinates. It acts as a conduit to transport the male gamete cells from the pollen grain—either from the stigma (in flowering plants) to the ovules at the base of the pistil or directly through ovule tissue in some gymnosperms. In maize, this single cell can grow longer than 12 inches (30 cm) to traverse the length of the pistil.
Pollen tubes were first discovered by Giovanni Battista Amici. They are used as a model for understanding plant cell behavior. Research is ongoing to comprehend how the pollen tube responds to extracellular guidance signals to achieve fertilization.
Description and history
Pollen tubes are produced by the male gametophytes of seed plants. They act as conduits to transport the male gamete cells from the pollen grain—either from the stigma (in flowering plants) to the ovules at the base of the pistil or directly through ovule tissue in some gymnosperms. Pollen tubes were first discovered by Giovanni Battista Amici.
Pollen tube formation is important for sexual reproduction to occur in seed plants. Plants have separate structures such as microsporocytes and megasporocytes and the pollen grains are transported to the female gametophyte through wind, water or pollinators. The last step before fertilization is the growth of the pollen tube to transfer non-motile sperm to the protected egg. Pollen tubes are unique to plants and their structure had evolved over plant history. The pollen tube formation is complex and the mechanism is not fully understood, but is of great interest to scientists.[1]
Angiosperms
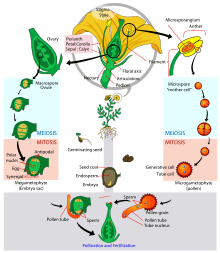
Pollen is produced by the stamen, the male reproductive organ of the flower. Each pollen grain contains a vegetative cell, and a generative cell that divides to form two sperm cells. The pollen is delivered by the opening of anthers for subsequent pollination (transfer of pollen grains to the pistil, the female reproductive organ). Transfer of pollen is usually carried out by wind, water, or animals . The ovaries hold the ovules that produce the female gamete: the egg cell, which waits in place for fertilization.
Once a pollen grain settles on a compatible pistil, it may germinate in response to a sugary fluid secreted by the mature stigma. Lipids at the surface of the stigma may also stimulate pollen tube growth for compatible pollen. Plants that are self-sterile often inhibit the pollen grains from their own flowers from growing pollen tubes. The presence of multiple grains of pollen has been observed to stimulate quicker pollen tube growth in some plants.[2] The vegetative cell then produces the pollen tube, a tubular protrusion from the pollen grain, which carries the sperm cells within its cytoplasm. The sperm cells are the male gametes that will join with the egg cell and the central cell in double fertilization.
The germinated pollen tube must then drill its way through the nutrient-rich style and curl to the bottom of the ovary to reach the ovule. Once the pollen tube reaches an ovule, it bursts to deliver the two sperm cells. One of the sperm fertilizes the egg cell which develops into an embryo, which will become the future plant. The other one fuses with both polar nuclei of the central cell to form the endosperm, which serves as the embryo's food supply. Finally, the ovary will develop into a fruit and the ovules will develop into seeds.
Gymnosperms
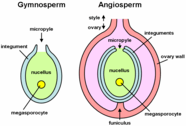
Gymnosperm pollen is produced in microsporangia borne on the scales of the male cone or microstrobilus. In most species the plants are wind-pollinated, and the pollen grains of conifers have air bladders that provide buoyancy in air currents. The grains are deposited in the micropyle of the ovule of a female cone or megastrobilus, where they mature for up to a year. In conifers and Gnetophytes the pollen germinate to produce a pollen tube that penetrates the megasporangium or nucellus carrying with it sperm nuclei that are transferred to the egg cell in the developing archegonia of the female plant.[3][4]
Mechanism of pollen tube growth
Recognition

The female sporophyte must recognize the pollen stuck to the stigma. Often, only pollen of the same species can successfully grow and outcrossed pollen is more successful in growing.[5][6] With self-incompatibility systems, outcrossed pollen grows and outcompetes self pollen. This is a gene level regulation in which gene loci of the gynoecium allow either self pollen to slowly grow, stop growing or burst while faster growth of outcrossed pollen occurs, increasing genetic diversity.[7] The main component is that this step determines which pollen grains are compatible to grow and parts of the style, and female embryo contribute in determining.[8] Using hybrid species and crossing pollen, the interaction with the style and pollen was found to influence growth of the pollen tube based on compatibility.[9]
Initiation
.jpg)
Pollen tube growth begins from when the pollen attaches to the stigma. It is followed by recognition and hydration of the pollen that allows for germination of the tube and ultimate growth.[10] In the pollen grain, the generative cell gives rise to the sperm, whereas the vegetative cells have a tube cell that grows the pollen tube. There is competition in this step as many pollen grains may compete to reach the egg. The stigma plays a role in guiding the sperm to a receptive ovule, in the case of many ovules.[10] Only compatible pollen grains are allowed to grow as determined by signaling with the stigma. Some plants have mechanisms in place to prevent selfing such as stigma and anther mature at different times or are different lengths, which significantly contributes to increasing genetic diversity of the next generation.[11][12]
There is great variation in the rate of growth of pollen tubes and many studies have focused on signaling.[11] The gene expression in the pollen grain has been identified as that of the gametophyte and not of the parental sporophyte, as it expresses its own unique mRNA and own enzymes.[11] In the peach plant, the style environment in which the pollen tube grows through, provides nutrition for the tube's growth to the egg.[10] Pollen tubes are tolerant and even pollen damaged by X-rays and gamma rays can still grow pollen tubes.[11]
Growth and Signaling
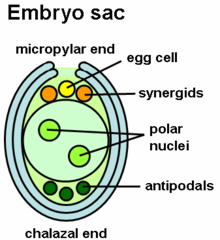
The interaction between the stigma-style and the pollen grain is a vital piece that contributes to the growth. The elongation of the tube is achieved with elongation of the cytoskeleton and it extends from the tip, which is regulated by high levels of calcium in the cytosol.[8] The calcium levels help the synaptic vesicles in the membranes grow and extend at the tip.[6] Polypeptides found in the style also regulate growth of tube and specific peptides that play a role in signaling for growth have been identified.
The LURE peptides that are secreted from the synergids, which occupy the space adjacent to the egg cell, can use attractants. Mutant Arabidopsis plant embryos were used and specifically in those without the synergids, the pollen tubes were unable to grow. The growth was also toward embryos of the same species as the pollen, so the intraspecific signaling helps fertilize egg and sperm of the same species. The signaling in the style is important as pollen tubes can grow without the presence of an embryo sac with just interaction with the style.[8][5] Other parts in the ovary include cytoplasmic factors like miRNA and chemical gradients that attract the tube to grow toward the synergids.[5][13]
Calcium and ethylene in Arabidopsis thaliana were involved in termination of the pollen tube when it grows near the ovary. The increase in calcium allowed release of the two sperm cells from the tube as well as degeneration of a synergid cell.[5] The chemical gradient of calcium can also contribute to termination early on in tube growth or at the appropriate time.[13]
The length of the pollen tube varies by species and it grows in an oscillating fashion until it is ready to release the sperm near the egg for fertilization to take place.[14][15] Some fast growing pollen tubes have been observed in lily, tobacco, and Impatiens sultanii. [15][16] The rate of growth confers advantage to the organism but it is not clear whether the variation in growth rate exists in the population or it has been selected for over generations due to increased fitness.[11]
Evolution
Many transitional features have been identified that show correlation between the evolution of the pollen tube with that of a non-motile sperm.[12] Early seed plants like ferns have spores and motile sperm that swim in a water medium, called zooidogamy.[17] The angiosperm pollen tube is simple, unbranched, and fast growing, however this is not the case for ancestral plants.
In gymnosperms like Gingko biloba and cycadophyta, a haustorial pollen tube forms. The tube simply soaks up nutrients from the female nucellus and grows in two stages.The pollen tube is highly branched and grows on the female sporophyte tissues. First, it grows the main tube followed by a more spherical tip at the end to allow the sperm to burst near the archegonia.[17] The binucleated, multiflagellated sperm can then swim to the egg.[12] Cycads have a less branched structured and the tip end swells the same way as in the gingko. In cycads, however, various enzymes have been identified in the pollen tube that direct growth and the nucellus tissues are more damaged with the tube growth.[17]
In other phyla of gymnosperms, coniferophyta and gnethophyta, the sperm is non motile, called siphonogamy and the pollen tube grows through the archegonia to help the sperm reach the egg more directly. Conifers can be branched or unbranched and they cause degeneration of the female tissue as it grows through more tissue.[17] Pines, for instance discharge cytoplasm of the sperm and union of the one sperm occurs as the other sperm degenerates. Yet, in gnethophyta, there are features more similar to angiosperm pollen tubes where the tube reaches the egg with an early form of double fertilization. However, the endosperm does not form and the second fertilization is aborted.[12]
In angiosperms, the mechanism has been studied more extensively as pollen tubes in flowering plants grow very fast through long styles to reach the well-protected egg. There is great variation in pollen tubes in angiosperms and many model plants like petunia, Arabidopsis, lily and tobacco plants have been studied for intraspecific variation and signaling mechanisms.[11] In flowering plants, a phenomenon called polyambry can occur where many ovules are fertilized and overall fitness of the organism is yet to be studied with respect to rate of pollen tube growth.[12][11]
Behavior
Cell physiology
Pollen tubes are an excellent model for the understanding of plant cell behavior.[18] They are easily cultivated in vitro and have a very dynamic cytoskeleton that polymerizes at very high rates, providing the pollen tube with interesting mechanical properties.[19] The pollen tube has an unusual kind of growth; it extends exclusively at its apex. Extending the cell wall only at the tip minimizes friction between the tube and the invaded tissue. This tip growth is performed in a pulsating manner rather than in a steady fashion.[20] The pollen tube’s journey through the style often results in depth-to-diameter ratios above 100:1 and up to 1000:1 in certain species. In maize, this single cell can grow longer than 12 inches (30 cm) to traverse the length of the pistil. The internal machinery and the external interactions that govern the dynamics of pollen tube growth are far from being fully understood.
Guidance
Extensive work has been dedicated to comprehend how the pollen tube responds to extracellular guidance signals to achieve fertilization.[21][18][22][23] Pollen tubes react to a combination of chemical, electrical, and mechanical cues during their journey through the pistil.[24][25][26] However, it is not clear how these external cues work or how they are processed internally. Moreover, sensory receptors for any external cue have not been identified yet. Nevertheless, several aspects have already been identified as central in the process of pollen tube growth. The actin filaments in the cytoskeleton, the peculiar cell wall, secretory vesicle dynamics, and the flux of ions, to name a few, are some of the fundamental features readily identified as crucial, but whose role has not yet been completely elucidated.
DNA repair
During pollen tube growth, DNA damages that arise need to be repaired in order for the male genomic information to be transmitted intact to the next generation. In the plant Cyrtanthus mackenii, bicellular mature pollen contains a generative cell and a vegetative cell.[27] Sperm cells are derived by mitosis of the generative cell during pollen tube elongation. The vegetative cell is responsible for pollen tube development. Double-strand breaks in DNA that arise appear to be efficiently repaired in the generative cell, but not in the vegetative cell, during the transport process to the female gametophyte.[27]
See also
References
- ↑ Li HJ, Meng JG, Yang WC (March 2018). "Multilayered signaling pathways for pollen tube growth and guidance". Plant Reproduction. 31 (1): 31–41. doi:10.1007/s00497-018-0324-7. PMID 29441420.
- ↑ O’Brien, Steven; et al. (1981). "Factors influencing pollen tube growth rate in angiosperms using Taraxucum as a model". Journal of Botanical Science. 14 (9): 156–178.
- ↑ Runions CJ, Owens JN (1999). "Sexual reproduction of interior spruce (Pinaceae). I. Pollen germination to archegonial maturation". International Journal of Plant Sciences. 160: 631–640.
- ↑ Runions CJ, Owens JM (1999). "Sexual reproduction of interior spruce (Pinaceae). II. Fertilization to early embryo formation". International Journal of Plant Sciences. 160: 641–652.
- 1 2 3 4 Kanaoka MM, Higashiyama T (December 2015). "Peptide signaling in pollen tube guidance". Current Opinion in Plant Biology. 28: 127–36. doi:10.1016/j.pbi.2015.10.006. PMID 26580200.
- 1 2 Derksen J, Rutten T, Amstel Tv, Win Ad, Doris F, Steer M (1995). "Regulation of pollen tube growth". Acta Botanica Neerlandica. 44 (2).
- ↑ Herrero M, Dickinson HG (February 1981). "Pollen tube development in Petunia hybrida following compatible and incompatible intraspecific matings". Journal of Cell Science. 47: 365–83. PMID 7263785.
- 1 2 3 Messerli MA, Créton R, Jaffe LF, Robinson KR (June 2000). "Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth". Developmental Biology. 222 (1): 84–98. doi:10.1006/dbio.2000.9709. PMID 10885748.
- ↑ Lewis D, Crowe LK (May 1958). "Unilateral interspecific incompatibility in flowering plants". Heredity. 12 (2): 233–256. doi:10.1038/hdy.1958.26. ISSN 1365-2540 – via nature.
- 1 2 3 Boavida LC, Vieira AM, Becker JD, Feijó JA (2005). "Gametophyte interaction and sexual reproduction: how plants make a zygote". The International Journal of Developmental Biology. 49 (5–6): 615–32. doi:10.1387/ijdb.052023lb. PMID 16096969.
- 1 2 3 4 5 6 7 Walsh NE, Charlesworth D (1992). "Evolutionary Interpretations of Differences in Pollen Tube Growth Rates". The Quarterly Review of Biology. 67 (1): 19–37. JSTOR 2830891.
- 1 2 3 4 5 Evert, R.F. (2013). Raven Biology of Plants. New York: W.H. Freeman and Co. pp. 430–456.
- 1 2 Shimizu KK, Okada K (October 2000). "Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance". Development. 127 (20): 4511–8. PMID 11003848.
- ↑ Abdelgadir, H.A; Johnson, S.D; Van Staden, J (2012-03-01). "Pollen viability, pollen germination and pollen tube growth in the biofuel seed crop Jatropha curcas (Euphorbiaceae)". South African Journal of Botany. 79: 132–139. doi:10.1016/j.sajb.2011.10.005. ISSN 0254-6299.
- 1 2 "Capturing Fast Pollen Tube Growth on Camera, Researchers Pin Down Plant Fertilization Process". Retrieved 2018-03-23.
- ↑ Bilderback DE (January 1981). "Impatiens pollen germination and tube growth as a bioassay for toxic substances". Environmental Health Perspectives. 37: 95–103. doi:10.1289/ehp.813795. PMC 1568632. PMID 7460890.
- 1 2 3 4 Friedman, William E (1993-01-01). "The evolutionary history of the seed plant male gametophyte". Trends in Ecology & Evolution. 8 (1): 15–21. doi:10.1016/0169-5347(93)90125-9. ISSN 0169-5347. PMID 21236093.
- 1 2 Malhó, Rui (2006). The pollen tube: a cellular and molecular perspective. Springer.
- ↑ Gossot O, Geitmann A (July 2007). "Pollen tube growth: coping with mechanical obstacles involves the cytoskeleton". Planta. 226 (2): 405–16. doi:10.1007/s00425-007-0491-5. PMID 17318608.
- ↑ Messerli MA, Créton R, Jaffe LF, Robinson KR (June 2000). "Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth". Developmental Biology. 222 (1): 84–98. doi:10.1006/dbio.2000.9709. PMID 10885748.
- ↑ Geitmann A, Palanivelu R (2007). "Fertilization requires communication: Signal generation and perception during pollen tube guidance". Floriculture and Ornamental Biotechnology. 1: 77–89.
- ↑ Malhó, Rui (1998). "Pollen tube guidance – the long and winding road". Sexual Plant Reproduction. 11 (5): 242–244. doi:10.1007/s004970050148.
- ↑ Okuda S, Higashiyama T (2010). "Pollen tube guidance by attractant molecules: LUREs". Cell Structure and Function. 35 (1): 45–52. doi:10.1247/csf.10003. PMID 20562497.
- ↑ Mascarenhas JP, Machlis L (January 1964). "Chemotropic Response of the Pollen of Antirrhinum majus to Calcium". Plant Physiology. 39 (1): 70–7. doi:10.1104/pp.39.1.70. PMC 550029. PMID 16655882.
- ↑ Robinson KR (December 1985). "The responses of cells to electrical fields: a review". The Journal of Cell Biology. 101 (6): 2023–7. doi:10.1083/jcb.101.6.2023. PMC 2114002. PMID 3905820.
- ↑ Chebli Y, Geitmann A (2007). "Mechanical principles governing pollen tube growth". Functional Plant Science and Biotechnology. 1: 232–245.
- 1 2 Hirano T, Takagi K, Hoshino Y, Abe T (2013). "DNA damage response in male gametes of Cyrtanthus mackenii during pollen tube growth". AoB PLANTS. 5: plt004. doi:10.1093/aobpla/plt004. PMC 3583183. PMID 23550213.
External links
- Pollen tube primer
- Images : Pollen tetrad and Pollen tube Calanthe discolor Lindl. - Flavon's Secret Flower Garden