Coelacanth
| Coelacanths | |
|---|---|
 | |
| Preserved specimen of West Indian Ocean coelacanth caught in 1974 off Salimani, Grand Comoro, Comoro Islands | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Sarcopterygii |
| Subclass: | Actinistia |
| Order: | Coelacanthiformes L. S. Berg, 1937 |
| Families and unplaced genera | |
| |
The coelacanths (/ˈsiːləkænθ/ (![]()
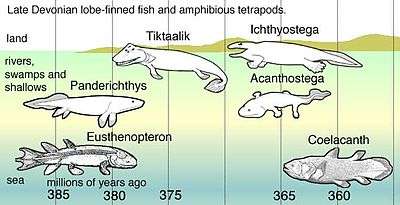
Coelacanths belong to the subclass Actinistia, a group of lobed-finned fish related to lungfish and certain extinct Devonian fish such as osteolepiforms, porolepiforms, rhizodonts, and Panderichthys.[5] Coelacanths were thought to have become extinct in the Late Cretaceous, around 66 million years ago, but were rediscovered in 1938 off the coast of South Africa.[6][7]
The coelacanth was long considered a "living fossil" because scientists thought it was the sole remaining member of a taxon otherwise known only from fossils, with no close relations alive,[5] and that it evolved into roughly its current form approximately 400 million years ago.[1] However, several recent studies have shown that coelacanth body shapes are much more diverse than previously thought.[8][9][10]
Etymology
The word Coelacanth is an adaptation of the Modern Latin Cœlacanthus ("hollow spine"), from the Greek κοῖλ-ος (koilos "hollow" + ἄκανθ-α akantha "spine"). It is a common name for the oldest living line of Sarcopterygii,[11] referring to the hollow caudal fin rays of the first fossil specimen described and named by Louis Agassiz in 1839.[5] The genus name Latimeria commemorates Marjorie Courtenay-Latimer who discovered the first specimen in a fish market.[12]
Discovery
The coelacanth, which is related to lungfishes and tetrapods, was believed to have been extinct since the end of the Cretaceous period.[13] More closely related to tetrapods than to the ray-finned fish, coelacanths were considered transitional species between fish and tetrapods.[14] On 23 December 1938, the first Latimeria specimen was found off the east coast of South Africa, off the Chalumna River (now Tyolomnqa).[6][15] Museum curator Marjorie Courtenay-Latimer discovered the fish among the catch of a local angler, Captain Hendrick Goosen.[6][15] A Rhodes University ichthyologist, J. L. B. Smith, confirmed the fish's importance with a famous cable: "MOST IMPORTANT PRESERVE SKELETON AND GILLS = FISH DESCRIBED."[6][15]
Its discovery 66 million years after it was believed to have gone extinct makes the coelacanth the best-known example of a Lazarus taxon, an evolutionary line that seems to have disappeared from the fossil record only to reappear much later. Since 1938, West Indian Ocean coelacanth have been found in the Comoros, Kenya, Tanzania, Mozambique, Madagascar, and in iSimangaliso Wetland Park, Kwazulu-Natal in South Africa.[16]
The Comoro Islands specimen was discovered in December 1952.[17] Between 1938 and 1975, 84 specimens were caught and recorded.[18]
The second extant species, Indonesian coelacanth, was described from Manado, North Sulawesi, Indonesia in 1999 by Pouyaud et al.[19] based on a specimen discovered by Mark V. Erdmann in 1998[20] and deposited at the Indonesian Institute of Sciences (LIPI).[21] Erdmann and his wife Arnaz Mehta first encountered a specimen at a local market in September 1997, but took only a few photographs of the first specimen of this species before it was sold. After confirming that it was a unique discovery, Erdmann returned to Sulawesi in November, 1997 interviewing fishermen to look for further examples. A second specimen was caught by a fisherman in July 1998 and it was then handed to Erdmann.[22][23]
The coelacanth has no real commercial value apart from being coveted by museums and private collectors. As a food fish it is almost worthless, as its tissues exude oils that give the flesh a foul flavor.[24] The coelacanth's continued survival may be threatened by commercial deep-sea trawling,[25] in which coelacanths are caught as bycatch.
Physical description


Coelacanths are a part of the clade Sarcopterygii, or the lobe-finned fishes. Externally, several characteristics distinguish the coelacanth from other lobe-finned fish. They possess a three-lobed caudal fin, also called a trilobate fin or a diphycercal tail. A secondary tail extending past the primary tail separates the upper and lower halves of the coelacanth. Cosmoid scales act as thick armor to protect the coelacanth's exterior. Several internal traits also aid in differentiating coelacanths from other lobe-finned fish. At the back of the skull, the coelacanth possesses a hinge, the intracranial joint, which allows it to open its mouth extremely wide. Coelacanths also retain an oil-filled notochord, a hollow, pressurized tube which is replaced by the vertebral column early in embryonic development in most other vertebrates.[26] The coelacanth heart is shaped differently from that of most modern fish, with its chambers arranged in a straight tube. The coelacanth braincase is 98.5% filled with fat; only 1.5% of the braincase contains brain tissue. The cheeks of the coelacanth are unique because the opercular bone is very small and holds a large soft-tissue opercular flap. A spiracular chamber is present, but the spiracle is closed and never opens during development.[27] Coelacanth also possess a unique rostral organ within the ethmoid region of the braincase.[5][28] Also unique to extant coelacanths is the presence of a "fatty lung" or a fat-filled single-lobed vestigial lung, homologous to other fishes' swim bladder. The parallel development of a fatty organ for buoyancy control suggest a unique specialization for deep-water habitats. There has also been discovered small, hard but flexible plates around the vestigial lung in adult specimen, though not around the fatty organ. The plates most likely had a regulation function for the volume of the lung.[29] Due to the size of the fatty organ, researchers assume it's responsible for the kidney's unusual relocation. The two kidneys, which are fused into one,[30] are located ventrally within the abdominal cavity, posterior to the cloaca.[31]
General description


Latimeria chalumnae and L. menadoensis are the only two known living coelacanth species.[5][32] The word "coelacanth" is derived from the Greek for “hollow spine”, because of the fish's unique hollow spine fins.[28] Coelacanths are large, plump, lobe-finned fish that can grow to more than 2 meters (6 feet 6 inches) and weigh around 90 kilograms (200 pounds). They are estimated to live for 60 years or more.[33] Modern coelacanths appear larger than those found as fossils.[34]
They are nocturnal piscivorous drift-hunters.[35] The body is covered in cosmoid scales that act as armor.[36] Coelacanths have eight fins – 2 dorsal fins, 2 pectoral fins, 2 pelvic fins, 1 anal fin and 1 caudal fin. The tail is very nearly equally proportioned and is split by a terminal tuft of fin rays that make up its caudal lobe. The eyes of the coelacanth are very large, while the mouth is very small. The eye is acclimatized to seeing in poor light by rods that absorb mostly short wavelengths. Coelacanth vision has evolved to a mainly blue-shifted color capacity.[37] Pseudomaxillary folds surround the mouth and replace the maxilla, a structure absent in coelacanths. Two nostrils, along with four other external openings, appear between the premaxilla and lateral rostral bones. The nasal sacs resemble those of many other fish and do not contain an internal nostril. The coelacanth's rostral organ, contained within the ethmoid region of the braincase, has three unguarded openings into the environment and is used as a part of the coelacanth's laterosensory system.[5] The coelacanth's auditory reception is mediated by its inner ear, which is very similar to that of tetrapods because it is classified as being a basilar papilla.[38]
Coelacanth locomotion is unique. To move around they most commonly take advantage of up- or down-wellings of current and drift. Their paired fins stabilize movement through the water. While on the ocean floor, they do not use the paired fins for any kind of movement. Coelacanths create thrust with their caudal fins for quick starts. Due to the abundance of its fins, the coelacanth has high maneuverability and can orient its body in almost any direction in the water. They have been seen doing headstands as well as swimming belly up. It is thought that the rostral organ helps give the coelacanth electroperception, which aids in movement around obstacles.[35]
DNA
In 2013, a group led by Chris Amemiya and Neil Shubin published the genome sequence of the coelacanth in the journal Nature. The African coelacanth genome was sequenced and assembled using DNA from a Comoros Islands Latimeria chalumnae specimen. It was sequenced by Illumina sequencing technology and assembled using the short read genome assembler ALLPATHS-LG.[39]
Due to their lobed fins and other features, it was once hypothesized that the coelacanth might be the most recent shared ancestor between terrestrial and marine vertebrates.[38][40] But after sequencing the full genome of the coelacanth, it was discovered that the lungfish is the most recent shared ancestor. Coelacanths and lungfish had already diverged from a common ancestor before the lungfish made the transition to land.[41]
The vertebrate land transition is one of the most important steps in our evolutionary history. We conclude that the closest living fish to the tetrapod ancestor is the lungfish, not the coelacanth. However, the coelacanth is critical to our understanding of this transition, as the lungfish have intractable genome sizes (estimated at 50–100Gb).[39]
Another important discovery made from the genome sequencing is that the coelacanths are still evolving today (but at a relatively slow rate). This raises questions about using the term "living fossil" to describe these creatures. While they were initially thought to be a prehistoric species that remained unchanged over millions of years, the discovery that they are still evolving, albeit slowly, causes some to question whether "living fossil" is an appropriate descriptor.[41]
One reason the coelacanths are evolving so slowly is the lack of evolutionary pressure on these organisms. They have few predators, and live deep in the ocean where conditions are very stable. Without much pressure for these organisms to adapt to survive, the rate at which they have evolved is much slower compared to other organisms.[41]
Taxonomy
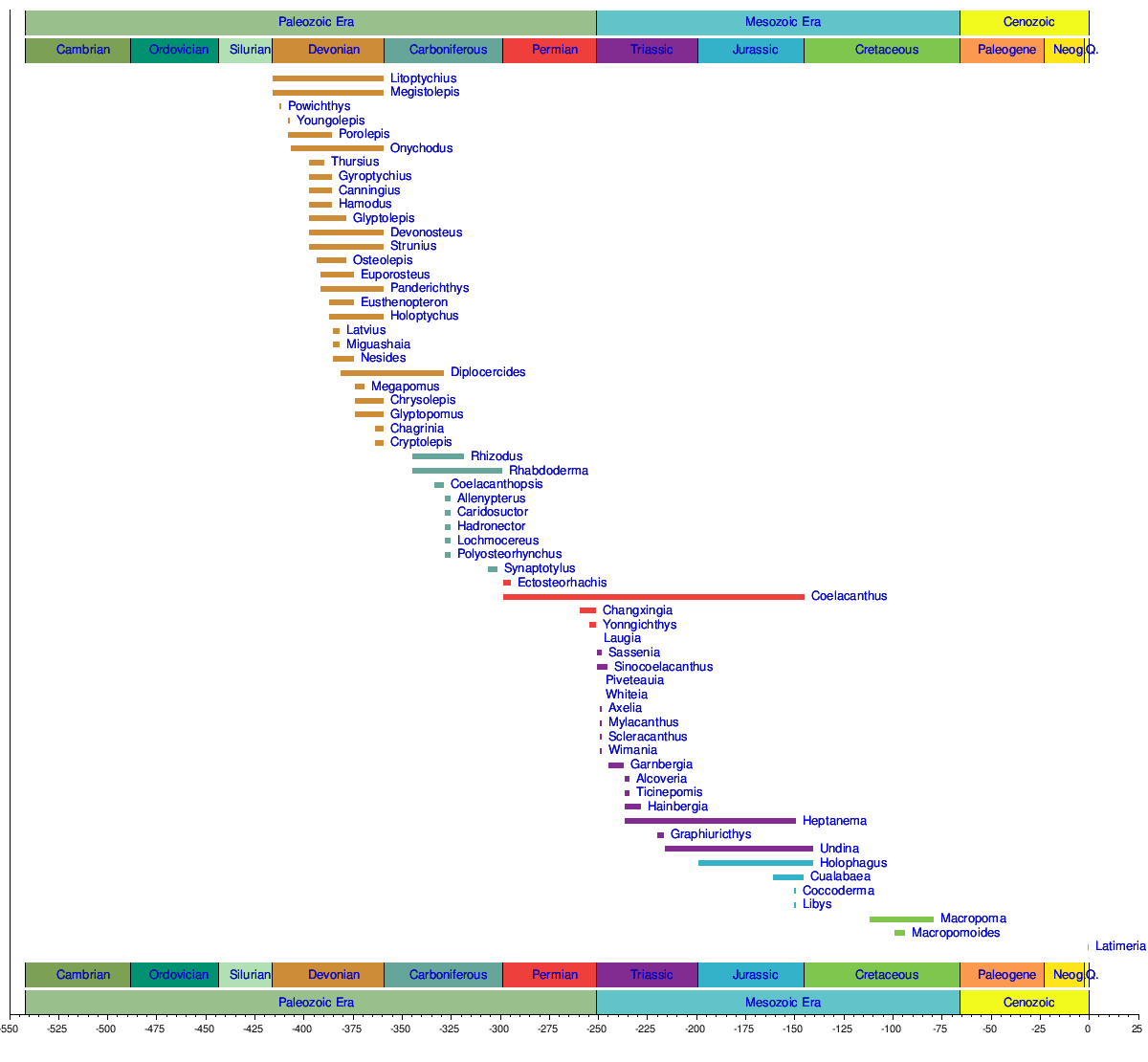
The following is a classification of known coelacanth genera and families:[5][10][32][42][43][44][45]
- Order Coelacanthiformes
- Family Whiteiidae (Triassic)
- Family Rebellatricidae (Triassic)
- Family Coelacanthidae (Permian to Cretaceous)
- Suborder Latimerioidei
- Family Mawsoniidae (Triassic to Jurassic)
- Family Latimeriidae L. S. Berg, 1940 (Triassic to Holocene)
- Holophagus
- Latimeria J. L. B. Smith, 1939
- Latimeria chalumnae J. L. B. Smith, 1939 (West Indian Ocean coelacanth)
- Latimeria menadoensis Pouyaud, Wirjoatmodjo, Rachmatika, Tjakrawidjaja, Hadiaty & Hadie, 1999 (Indonesian coelacanth)
- Libys
- Macropoma
- Macropomoides
- Ticinepomis
- Foreyia
- Swenzia
- Undina
- Megacoelacanthus
Fossil record
According to genetic analysis of current species, the divergence of coelacanths, lungfish and tetrapods is thought to have occurred about 390 million years ago.[1] Coelacanths were once thought to have undergone extinction 66 million years ago during the Cretaceous–Paleogene extinction event. The first recorded coelacanth fossil, found in Australia, was of a jaw that dated back 360 million years, named Eoachtinistia foreyi. The most recent genus of coelacanth in the fossil record is Megalocoelacanthus, whose disarticulated remains are found in Campanian to possibly earliest Maastrichtian-aged marine strata of the Eastern and Central United States.[46][47] The fossil record is unique because coelacanth fossils were found 100 years before the first live specimen was identified. In 1938, Courtenay-Latimer rediscovered the first live specimen, L. chalumnae, caught off the coast of East London, South Africa. In 1997, a marine biologist on honeymoon discovered the second live species, Latimeria menadoensis, in an Indonesian market.
In July 1998, the first live specimen of Latimeria menadoensis was caught in Indonesia. Approximately 80 species of coelacanth have been described, including the two extant species. Before the discovery of a live specimen, the coelacanth time range was thought to have spanned from the Middle Devonian to the Upper Cretaceous period. Although fossils found during that time were claimed to demonstrate a similar morphology,[4][5] recent studies have expressed the view that coelacanth morphologic conservatism is a belief not based on data.[8][9][10][48]
The following cladogram is based on multiple sources.[10][43][44][45]
| Coelacanthiformes |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Timeline of genera
Geographic distribution
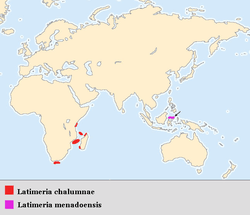
The current coelacanth range is primarily along the eastern African coast, although Latimeria menadoensis was discovered off Indonesia. Coelacanths have been found in the waters of Kenya, Tanzania, Mozambique, South Africa, Madagascar, Comoros and Indonesia.[4] Most Latimeria chalumnae specimens that have been caught have been captured around the islands of Grande Comore and Anjouan in the Comoros Archipelago (Indian Ocean). Though there are cases of L. chalumnae caught elsewhere, amino acid sequencing has shown no big difference between these exceptions and those found around Comore and Anjouan. Even though these few may be considered strays, there are several reports of coelacanths being caught off of the coast of Madagascar. This leads scientists to believe that the endemic range of Latimeria chalumnae coelacanths stretches along the eastern coast of Africa from the Comoros Islands, past the western coast of Madagascar to the South African coastline.[5] Mitochondrial DNA sequencing of coelacanths caught off the coast of southern Tanzania suggests a divergence of the two populations some 200,000 years ago. This could refute the theory that the Comoros population is the main population while others represent recent offshoots.[49]
The geographical range of the Indonesia coelacanth, Latimeria menadoensis, is believed to be off the coast of Manado Tua Island, Sulawesi, Indonesia in the Celebes Sea.[3] Key components confining coelacanths to these areas are food and temperature restrictions, as well as ecological requirements such as caves and crevices that are well-suited for drift feeding.[50] Teams of researchers using submersibles have recorded live sightings of the fish in the Sulawesi Sea as well as in the waters of Biak in Papua.[51][52]
Ecology
Anjouan Island and the Grande Comore provide ideal underwater cave habitats for coelacanths. The islands' underwater volcanic slopes, steeply eroded and covered in sand, house a system of caves and crevices which allow coelacanths resting places during the daylight hours. These islands support a large benthic fish population that help to sustain coelacanth populations.[50][53]
During the daytime, coelacanths rest in caves anywhere from 100 to 500 meters deep. Others migrate to deeper waters.[4][5] The cooler waters (below 120 meters) reduce the coelacanths' metabolic costs. Drifting toward reefs and night feeding saves vital energy.[50] Resting in caves during the day also saves energy otherwise used to fight currents.[53]
Coelacanths are nocturnal piscivores who feed mainly on benthic fish populations and various cephalopods. They are "passive drift feeders", slowly drifting currents with only minimal self-propulsion, eating whatever prey they encounter.[50][53]
Coelacanths are fairly peaceful when encountering others of their kind, remaining calm even in a crowded cave. They do avoid body contact, however, withdrawing immediately if contact occurs. When approached by foreign potential predators (e.g. a submersible), they show panic flight reactions, suggesting that coelacanths are most likely prey to large deepwater predators. Shark bite marks have been seen on coelacanths; sharks are common in areas inhabited by coelacanths.[53] Electrophoresis testing of 14 coelacanth enzymes shows little genetic diversity between coelacanth populations. Among the fish that have been caught were about equal numbers of males and females.[5] Population estimates range from 210 individuals per population all the way to 500 per population.[5][54] Because coelacanths have individual color markings, scientists think that they recognize other coelacanths via electric communication.[53]
Life history

Coelacanths are ovoviviparous, meaning that the female retains the fertilized eggs within her body while the embryos develop during a gestation period of over a year. Typically, females are larger than the males; their scales and the skin folds around the cloaca differ. The male coelacanth has no distinct copulatory organs, just a cloaca, which has a urogenital papilla surrounded by erectile caruncles. It is hypothesized that the cloaca everts to serve as a copulatory organ.[5][7]
Coelacanth eggs are large with only a thin layer of membrane to protect them. Embryos hatch within the female and eventually are given live birth, which is a rarity in fish. This was only discovered when the American Museum of Natural History dissected its first coelacanth specimen in 1975 and found it pregnant with five embryos.[55] Young coelacanths resemble the adult, the main differences being an external yolk sac, larger eyes relative to body size and a more pronounced downward slope of the body. The juvenile coelacanth's broad yolk sac hangs below the pelvic fins. The scales and fins of the juvenile are completely matured; however, it does lack odontodes, which it gains during maturation.[7]
A study that assessed the paternity of the embryos inside two Coelacanth females indicated that each clutch was sired by a single male.[56] This could mean that females mate monandrously, i.e. with one male only. Polyandry, female mating with multiple males, is common in both plants and animals and can be advantageous (e.g. insurance against mating with an infertile or incompatible mate), but also confers costs (increased risk of infection, danger of falling prey to predators, increased energy input when searching for new males). Alternatively, the study's results could indicate that, despite female polyandry, one male is used to fertilise all the eggs, potentially through female sperm choice or last-male sperm precedence.
Conservation
Because little is known about the coelacanth, the conservation status is difficult to characterize. According to Fricke et al. (1995), there should be some stress put on the importance of conserving this species. From 1988 to 1994, Fricke counted some 60 individuals of L. chalumnae on each dive. In 1995 that number dropped to 40. Even though this could be a result of natural population fluctuation, it also could be a result of overfishing. The IUCN currently classifies L. chalumnae as Critically Endangered,[57] with a total population size of 500 or fewer individuals.[5] L. menadoensis is considered Vulnerable, with a significantly larger population size (fewer than 10,000 individuals).[58]
Currently, the major threat towards the coelacanth is the accidental capture by fishing operations.[59] Coelacanths usually are caught when local fishermen are fishing for oilfish. Fishermen sometimes snag a coelacanth instead of an oilfish because they traditionally fish at night, when oilfish (and coelacanths) feed. Before scientists became interested in coelacanths, they were thrown back into the water if caught. Now that there is an interest in them, fishermen trade them in to scientists or other officials once they have been caught. Before the 1980s, this was a problem for coelacanth populations. In the 1980s, international aid gave fiberglass boats to the local fishermen, which resulted in fishing beyond the coelacanth territories into more fish-productive waters. Since then, most of the motors on the boats have broken down so the local fishermen are now back in the coelacanth territory, putting the species at risk again.[5][60]
Different methods to minimize the number of coelacanths caught include moving fishers away from the shore, using different laxatives and malarial salves to reduce the quantity of oilfish needed, using coelacanth models to simulate live specimens, and increasing awareness of the need to protect the species. In 1987 the Coelacanth Conservation Council advocated the conservation of coelacanths. The CCC has many branches of its agency located in Comoros, South Africa, Canada, the United Kingdom, the U.S., Japan and Germany. The agencies were established to help protect and encourage population growth of coelacanths.[5][61]
A "Deep Release Kit" was developed in 2014 and distributed by private initiative, consisting of a weighted hook assembly that allows a fisherman to return an accidentally caught coelacanth to deep waters where the hook can be detached once it hits the sea floor. Conclusive reports about the effectiveness of this method are still pending.[62]
In 2002, the South African Coelacanth Conservation and Genome Resource Programme was launched to help further the studies and conservation of the coelacanth. This program focuses on biodiversity conservation, evolutionary biology, capacity building, and public understanding. The South African government committed to spending R10 million on the program.[63][64] In 2011, a plan for a Tanga Coelacanth Marine Park was designed to conserve marine biodiversity for marine animals including the coelacanth. The park was designed to reduce habitat destruction and improve prey availability for endangered species.[65]
Human consumption
Coelacanths are considered a poor source of food for humans and likely most other fish-eating animals. Coelacanth flesh has high amounts of oil, urea, wax esters, and other compounds that are difficult to digest and can cause diarrhea. Their scales themselves emit mucus, which combined with the excessive oil their bodies produce, make coelacanths a slimy food.[66] Where the coelacanth is more common, local fishermen avoid it because of its potential to sicken consumers.[67]
References
- 1 2 3 Johanson, Z.; Long, J. A; Talent, J. A; Janvier, P.; Warren, J. W (2006). "Oldest coelacanth, from the Early Devonian of Australia". Biology Letters. 2 (3): 443–6. doi:10.1098/rsbl.2006.0470. PMC 1686207. PMID 17148426.
- ↑ "Coelacanths, Coelacanth Pictures, Coelacanth Facts – National Geographic". National Geographic. Retrieved 2015-10-30.
- 1 2 Holder, Mark T.; Erdmann, Mark V.; Wilcox, Thomas P.; Caldwell, Roy L.; Hillis, David M. (1999). "Two Living Species of Coelacanths?". Proceedings of the National Academy of Sciences of the United States of America. 96 (22): 12616–20. Bibcode:1999PNAS...9612616H. doi:10.1073/pnas.96.22.12616. JSTOR 49396. PMC 23015. PMID 10535971.
- 1 2 3 4 Butler, Carolyn (March 2011). "Living Fossil Fish". National Geographic: 86–93.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Forey, Peter L (1998). History of the Coelacanth Fishes. London: Chapman & Hall. ISBN 978-0-412-78480-4.
- 1 2 3 4 Smith, J. L. B. (1956). Old Fourlegs: the Story of the Coelacanth. Longmans Green.
- 1 2 3 Lavett Smith, C.; Rand, Charles S.; Schaeffer, Bobb; Atz, James W. (1975). "Latimeria, the Living Coelacanth, is Ovoviviparous". Science. 190 (4219): 1105–6. Bibcode:1975Sci...190.1105L. doi:10.1126/science.190.4219.1105.
- 1 2 Friedman, Matt; Coates, Michael I.; Anderson, Philip (2007). "First discovery of a primitive coelacanth fin fills a major gap in the evolution of lobed fins and limbs". Evolution & Development. 9 (4): 329–37. doi:10.1111/j.1525-142X.2007.00169.x. PMID 17651357.
- 1 2 Friedman, Matt; Coates, Michael I. (2006). "A newly recognized fossil coelacanth highlights the early morphological diversification of the clade". Proceedings of the Royal Society B: Biological Sciences. 273 (1583): 245–50. doi:10.1098/rspb.2005.3316. JSTOR 25223279. PMC 1560029. PMID 16555794.
- 1 2 3 4 Wendruff, Andrew J.; Wilson, Mark V. H. (2012). "A fork-tailed coelacanth, Rebellatrix divaricerca, gen. Et sp. Nov. (Actinistia, Rebellatricidae, fam. Nov.), from the Lower Triassic of Western Canada". Journal of Vertebrate Paleontology. 32 (3): 499–511. doi:10.1080/02724634.2012.657317.
- ↑ "The Coelancanth | Actforlibraries.org". www.actforlibraries.org. Retrieved 2015-10-30.
- ↑ "Smithsonian Institution – The Coelacanth: More Living than Fossil". vertebrates.si.edu. Retrieved 2015-10-30.
- ↑ "Coelacanth – Deep Sea Creatures on Sea and Sky". www.seasky.org. Retrieved 2015-10-27.
- ↑ Meyer, Axel. "Molecular evidence on the origin of tetrapods and the relationships of the coelacanth". Trends in Ecology & Evolution. 10 (3): 111–116. doi:10.1016/s0169-5347(00)89004-7.
- 1 2 3 "'Discovery' of the Coelacanth".
- ↑ Venter, P.; Timm, P.; Gunn, G.; le Roux, E.; Serfontein, C. (2000). "Discovery of a viable population of coelacanths (Latimeria chalumnae Smith, 1939) at Sodwana Bay, South Africa". South African Journal of Science. 96 (11/12): 567–568.
- ↑ "Prehistoric fish offers rare glimpse of hidden sea life - Coelacanth (1953)". Abilene Reporter-News. 1953-02-23. p. 25. Retrieved 2017-06-18.
- ↑ "70-million-year-old fish dissected - Coaelacanth (1975)". Redlands Daily Facts. 1975-05-28. p. 6. Retrieved 2017-06-18.
- ↑ Pouyaud, Laurent; Wirjoatmodjo, Soetikno; Rachmatika, Ike; Tjakrawidjaja, Agus; Hadiaty, Renny; Hadie, Wartono (1999). "Une nouvelle espèce de cœlacanthe. Preuves génétiques et morphologiques" [A new species of coelacanth. Genetic and morphologic proof]. Comptes Rendus de l'Académie des Sciences (in French). 322 (4): 261–7. Bibcode:1999CRASG.322..261P. doi:10.1016/S0764-4469(99)80061-4. PMID 10216801.
- ↑ Erdmann, Mark V.; Caldwell, Roy L.; Moosa, M. Kasim (1998). "Indonesian 'king of the sea' discovered". Nature. 395 (6700): 335. Bibcode:1998Natur.395..335E. doi:10.1038/26376.
- ↑ Holden, Constance (March 30, 1999). "Dispute Over a Legendary Fish". Science.
- ↑ Gee, Henry (1 October 1998). "Coelacanth discovery in Indonesia". Nature. doi:10.1038/news981001-1.
- ↑ "The Discovery". University of California Museum of Paleontology.
- ↑ Piper, Ross (2007). Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals. Greenwood Press. ISBN 978-0-313-33922-6.
- ↑ Gilmore, Inigo (7 January 2006). "Dinosaur fish pushed to the brink by deep-sea trawlers". The Observer.
- ↑ What do we know about the coelacanths – Science in Africa Archived 21 September 2013 at the Wayback Machine.
- ↑ Gaining Ground, Second Edition: The Origin and Evolution of Tetrapods
- 1 2 "The Coelacanth – a Morphological Mixed Bag". ReefQuest Centre for Shark Research.
- ↑ Brito, Paulo M.; Meunier, François J.; Clément, Gael; Geffard-Kuriyama, Didier (2010). "The histological structure of the calcified lung of the fossil coelacanth Axelrodichthys araripensis (Actinistia: Mawsoniidae)". Palaeontology. 53 (6): 1281–90. doi:10.1111/j.1475-4983.2010.01015.x.
- ↑ From fish to philosopher
- ↑ History of the Coelacanth Fishes
- 1 2 Nelson, Joseph S. (2006). Fishes of the World. Hoboken, New Jersey: John Wiley. ISBN 978-0-471-75644-6.
- ↑ "Coelacanths, Coelacanth Pictures, Coelacanth Facts – National Geographic". National Geographic. Retrieved 2015-10-28.
- ↑ "Coelacanth – body, used, Earth, form, animals, part, Anextinctdiscovery". www.scienceclarified.com. Retrieved 2015-10-30.
- 1 2 Fricke, Hans; Reinicke, Olaf; Hofer, Heribert; Nachtigall, Werner (1987). "Locomotion of the coelacanth Latimeria chalumnae in its natural environment". Nature. 329 (6137): 331–3. Bibcode:1987Natur.329..331F. doi:10.1038/329331a0.
- ↑ Sherman, Vincent R. (2016). "A comparative study of piscine defense: The scales of Arapaima gigas, Latimeria chalumnae and Atractosteus spatula". Journal of the Mechanical Behavior of Biomedical Materials. doi:10.1016/j.jmbbm.2016.10.001.
- ↑ Yokoyama, Shozo; Zhang, Huan; Radlwimmer, F. Bernhard; Blow, Nathan S. (1999). "Adaptive Evolution of Color Vision of the Comoran Coelacanth (Latimeria chalumnae)". Proceedings of the National Academy of Sciences of the United States of America. 96 (11): 6279–84. Bibcode:1999pnas...96.6279y. doi:10.1073/pnas.96.11.6279. JSTOR 47861. PMC 26872. PMID 10339578.
- 1 2 Fritzsch, B. (1987). "Inner ear of the coelacanth fish Latimeria has tetrapod affinities". Nature. 327 (6118): 153–4. Bibcode:1987Natur.327..153F. doi:10.1038/327153a0. PMID 22567677.
- 1 2 Amemiya, Chris T.; Alföldi, Jessica; Lee, Alison P.; Fan, Shaohua; Philippe, Hervé; MacCallum, Iain; Braasch, Ingo; Manousaki, Tereza; Schneider, Igor; et al. (18 April 2013). "The African coelacanth genome provides insights into tetrapod evolution" (PDF). Nature. 496 (7445): 311–6. Bibcode:2013Natur.496..311A. doi:10.1038/nature12027. PMC 3633110. PMID 23598338. Retrieved 2013-04-20.
- ↑ Northcutt, RG (1987). "Lungfish neural characters and their bearing on sareopterygian phylogeny". J Morph Suppl 1: 277–297.
- 1 2 3 http://www.smithsonianmag.com/science-nature/dna-sequencing-reveals-that-coelacanths-werent-the-missing-link-between-sea-and-land-25025860/?no-ist
- ↑ Nelson, Joseph S. (2006). Fishes of the World. John Wiley & Sons. p. 601. ISBN 0-471-25031-7.
- 1 2 Gallo, V. & M.S.S. de Carvalho, H.R.S. Santos (2010). "New occurrence of †Mawsoniidae (Sarcopterygii, Actinistia) in the Morro do Chaves Formation, Lower Cretaceous of the Sergipe-Alagoas Basin, Northeastern Brazil". Boletim do Museu Paraense Emílio Goeldi. 5 (2): 195–205.
- 1 2 Long, J. A. (1995). The rise of fishes: 500 million years of evolution. Baltimore: Johns Hopkins University Press.
- 1 2 Cloutier, R.; Ahlberg, P. E. (1996). Morphology, characters, and the interrelationships of basal sarcopterygians. pp. 445–79.
- ↑ Schwimmer, David R., J. D. Stewart, and G. Dent Williams. "Giant fossil coelacanths of the Late Cretaceous in the eastern United States." Geology 22.6 (1994): 503-506.
- ↑ Gottfried, Michael D., Raymond R. Rogers, and K. Curry Rogers. "First record of Late Cretaceous coelacanths from Madagascar." Recent advances in the origin and early radiation of vertebrates (2004): 687-691.
- ↑ Casane, Didier; Laurenti, Patrick (2013). "Why coelacanths are not 'living fossils'". BioEssays. 35 (4): 332–8. doi:10.1002/bies.201200145. PMID 23382020.
- ↑ Nikaido, Masato; Sasaki, Takeshi; Emerson, J. J.; Aibara, Mitsuto; Mzighani, Semvua I.; Budeba, Yohana L.; Ngatunga, Benjamin P.; Iwata, Masamitsu; Abe, Yoshitaka (2011-11-01). "Genetically distinct coelacanth population off the northern Tanzanian coast". Proceedings of the National Academy of Sciences. 108 (44): 18009–18013. Bibcode:2011PNAS..10818009N. doi:10.1073/pnas.1115675108. ISSN 0027-8424. PMC 3207662. PMID 22025696.
- 1 2 3 4 Fricke, H.; Plante, R. (1988). "Habitat requirements of the living coelacanth Latimeria chalumnae at grande comore, Indian Ocean". Naturwissenschaften. 75 (3): 149–51. Bibcode:1988NW.....75..149F. doi:10.1007/BF00405310.
- ↑ Augy Syaihailatua (March 30, 2015). "Hunting for living fossils in Indonesian waters". The Conversation.
- ↑ Rik Nulens, Lucy Scott and Marc Herbin (22 September 2011). "An updated inventory of all known specimens of the coelacanth, Latimeria spp" (PDF). Smithiana.
- 1 2 3 4 5 Fricke, Hans; Schauer, Jürgen; Hissmann, Karen; Kasang, Lutz; Plante, Raphael (1991). "Coelacanth Latimeria chalumnae aggregates in caves: First observations on their resting habitat and social behavior". Environmental Biology of Fishes. 30 (3): 281–6. doi:10.1007/BF02028843.
- ↑ Hissmann, Karen; Fricke, Hans; Schauer, Jürgen (2008). "Population Monitoring of the Coelacanth (Latimeria chalumnae)". Conservation Biology. 12 (4): 759–65. doi:10.1111/j.1523-1739.1998.97060.x. JSTOR 2387536.
- ↑ "The Coelacanth: Five Fast Facts". AMNH. Retrieved 2015-10-28.
- ↑ "Single-male paternity in coelacanths". www.nature.com. Retrieved 2016-06-07.
- ↑ "Latimeria chalumnae". IUCN Red List of Threatened Species.
- ↑ "Latimeria menadoensis". IUCN Red List of Threatened Species.
- ↑ "Coelacanth". Animal Planet. Retrieved 2015-10-29.
- ↑ Fricke, Hans; Hissmann, Karen; Schauer, Jürgen; Plante, Raphael (1995). "Yet more danger for coelacanths". Nature. 374 (6520): 314–5. Bibcode:1995Natur.374..314F. doi:10.1038/374314a0.
- ↑ Commerce, Protected Resources Webmaster, Office of Protected Resources, NOAA Fisheries, U.S. Department of. "Redirect – Office of Protected Resources – NOAA Fisheries" (PDF). www.nmfs.noaa.gov. Retrieved 2015-10-30.
- ↑ "Proposed Rule To List the Tanzanian DPS of African Coelacanth as Threatened Under the Endangered Species Act". NOOA (US). Retrieved 30 October 2015.
- ↑ "South Africa announces plans for Coelacanth Programme" (Press release). Science in Africa. February 2002. Retrieved 19 April 2013.
- ↑ "South African Coelacanth Conservation and Genome Resource Programme". African Conservation Foundation.
- ↑ Commerce, Protected Resources Webmaster, Office of Protected Resources, NOAA Fisheries, U.S. Department of. "Redirect – Office of Protected Resources – NOAA Fisheries" (PDF). www.nmfs.noaa.gov. Retrieved 2015-10-30.
- ↑ "The Creature Feature: 10 Fun Facts About the Coelacanth". WIRED. Retrieved 2015-10-30.
- ↑ Adams, Cecil (30 December 2011). "Know any good recipes for endangered prehistoric fish? Plus: Do caribou like the Alaska oil pipeline?". The Straight Dope.
Further reading
- Smith, J. L. B. (1956). Old Fourlegs: the Story of the Coelacanth. Longmans Green.
- Fricke, Hans (June 1988). "Coelacanths — The Fish That Time Forgot". National Geographic. Vol. 173 no. 6. pp. 824–838. ISSN 0027-9358. OCLC 643483454.
- Wade, Nicholas (18 April 2013). "Fish's DNA May Explain How Fins Turned to Feet". The New York Times. pp. A3.
- Thomson, Keith S. (1991). Living Fossil: the Story of the Coelacanth. W. W. Norton.
- Sepkoski, Jack (2002). "A compendium of fossil marine animal genera". Bulletins of American Paleontology. 364: 560. Archived from the original on 20 February 2009. Retrieved 2011-05-17.
- Weinberg, Samantha (1999). A Fish Caught In Time: The Search for the Coelacanth. Fourth Estate.
- Bruton, Mike (2015). When I Was a Fish: Tales of an Ichthyologist. Jacana Media(Pty)Ltd.
External links
| Wikimedia Commons has media related to Coelacanthiformes. |
| Wikispecies has information related to Latimeria |
- Anatomy of the coelacanth by PBS (Adobe Flash required)
- Dinofish.com (requires a frame-capable browser)
- Butler, Carolyn (August 2012). "Der Quastenflosser: Ein Fossil taucht auf" [The Coelacanth: A fossil turns up]. National Geographic Deutschland (in German).
- Amemiya, Chris T.; Alföldi, Jessica; Lee, Alison P.; Fan, Shaohua; Philippe, Hervé; MacCallum, Iain; Braasch, Ingo; Manousaki, Tereza; Schneider, Igor; et al. (2013). "The African coelacanth genome provides insights into tetrapod evolution". Nature. 496 (7445): 311–6. Bibcode:2013Natur.496..311A. doi:10.1038/nature12027. PMC 3633110. PMID 23598338.
- 'Living fossil' coelacanth genome sequenced BBC News Science & Environment; 17 April 2013

