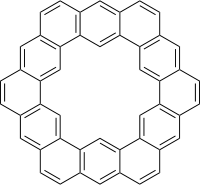
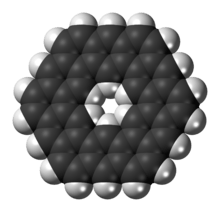
Kekulene
 | |
 | |
 | |
| Names | |
|---|---|
| Other names
[12]–Coronaphen, [12]Circulene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C48H24 | |
| Molar mass | 600.72 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Kekulene is a polycyclic aromatic hydrocarbon and a circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.
References
- Peter, R.; Jenny, W. (1966). "250. Höhere, kondensierte Ringsysteme. 1. Mitteilung [1]: Untersuchungen in der [10]-Coronaphenreihe: Synthese von Di-[benzo(c)phenanthren-3, 10-dimethylen]". Helvetica Chimica Acta. 49 (7): 2123. doi:10.1002/hlca.660490717.
- Staab, Heinz A.; Diederich, François (1983). "Cycloarenes, a New Class of Aromatic Compounds, I. Synthesis of Kekulene". Chemische Berichte. 116 (10): 3487. doi:10.1002/cber.19831161021.
- Krieger, Claus; Diederich, Francois; Schweitzer, Dieter; Staab, Heinz A. (1979). "Molecular Structure and Spectroscopic Properties of Kekulene". Angewandte Chemie International Edition in English. 18 (9): 699. doi:10.1002/anie.197906991.
- Aihara, Junichi (1992). "Is superaromaticity a fact or an artifact? The kekulene problem". Journal of the American Chemical Society. 114 (3): 865. doi:10.1021/ja00029a009.
- Diederich, François; Staab, Heinz A. (1978). "Benzenoid versus Annulenoid Aromaticity: Synthesis and Properties of Kekulene". Angewandte Chemie International Edition in English. 17 (5): 372. doi:10.1002/anie.197803721.
- Jiao, Haijun; Schleyer, Paul von Ragué (1996). "Is Kekulene Really Superaromatic?". Angewandte Chemie International Edition in English. 35 (20): 2383. doi:10.1002/anie.199623831.
- Schweitzer, D.; Hausser, K.H.; Vogler, H.; Diederich, F.; Staab, H.A. (1982). "Electronic properties of kekulene". Molecular Physics. 46 (5): 1141. doi:10.1080/00268978200101861.
- Cioslowski, Jerzy; O'Connor, Peter B.; Fleischmann, Eugene D. (1991). "Is superbenzene superaromatic?". Journal of the American Chemical Society. 113 (4): 1086. doi:10.1021/ja00004a005.
- Staab, Heinz A.; Diederich, FrançOis; Krieger, Claus; Schweitzer, Dieter (1983). "Cycloarenes, a New Class of Aromatic Compounds, II. Molecular Structure and Spectroscopic Properties of Kekulene". Chemische Berichte. 116 (10): 3504. doi:10.1002/cber.19831161022.
- Zhou, Zhongxiang (1995). "Are kekulene, coronene, and corannulene tetraanion superaromatic? Theoretical examination using hardness indices". Journal of Physical Organic Chemistry. 8 (2): 103. doi:10.1002/poc.610080209.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.