Sterane
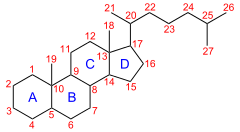
Sterane (cyclopentanoperhydrophenanthrenes or cyclopentane perhydro phenanthrene) compounds are a class of 4-cyclic compounds derived from steroids or sterols via diagenetic and catagenetic degradation and saturation. Steranes have an androstane skeleton with a side chain at carbon C-17. The sterane structure constitutes the core of all sterols. Steranes are sometimes used as biomarkers for the presence of eukaryotic cells. [1]
Steranes may be rearranged to diasteranes during diagenesis (C-27 to C-30, rearrangement at C-18 and C-19, no R at C-24). Oils from clastic source rocks tend to be rich in diasteranes.
Cholesterol and its derivatives (such as progesterone, aldosterone, cortisol, and testosterone), are common examples of compounds with the cyclopentanoperhydrophenanthrene nucleus.
See also
References
- ↑ About biomarkers geobiology@mit. Accessed 8 October 2009.