Potassium sulfate
 Arcanite | |
 | |
| Names | |
|---|---|
| Other names
Potassium sulphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.013 |
| E number | E515 (acidity regulators, ...) |
| KEGG | |
PubChem CID |
|
| RTECS number | TT5900000 |
| UNII | |
| |
| |
| Properties | |
| K2SO4 | |
| Molar mass | 174.259 g/mol |
| Appearance | White solid |
| Odor | odorless |
| Density | 2.66 g/cm3[1] |
| Melting point | 1,069[2] °C (1,956 °F; 1,342 K) |
| Boiling point | 1,689 °C (3,072 °F; 1,962 K) |
| 111 g/L (20 °C) 120 g/L (25 °C) 240 g/L (100 °C) | |
| Solubility | slightly soluble in glycerol insoluble in acetone, alcohol, CS2 |
| −67.0·10−6 cm3/mol | |
Refractive index (nD) |
1.495 |
| Structure | |
| orthorhombic | |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | External MSDS |
| R-phrases (outdated) | R22 |
| S-phrases (outdated) | S36 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
6600 mg/kg (oral, rat)[3] |
| Related compounds | |
Other anions |
Potassium selenate Potassium tellurate |
Other cations |
Lithium sulfate Sodium sulfate Rubidium sulfate Caesium sulfate |
Related compounds |
Potassium hydrogen sulfate Potassium sulfite Potassium bisulfite Potassium persulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium sulfate (K2SO4) (in British English potassium sulphate, also called sulphate of potash, arcanite, or archaically known as potash of sulfur) is a non-flammable white crystalline salt which is soluble in water. The chemical compound is commonly used in fertilizers, providing both potassium and sulfur.
When potassium sulfate is heated in water and subjected to swirling in a beaker, the crystals form a multi-arm spiral structure when allowed to settle.[4] Potassium sulfate could be used to study spiral structures in the laboratory.
History
Potassium sulfate (K2SO4) has been known since early in the 14th century, and it was studied by Glauber, Boyle, and Tachenius. In the 17th century, it was named arcanuni or sal duplicatum, as it was a combination of an acid salt with an alkaline salt. It was also known as vitriolic tartar and Glaser's salt or sal polychrestum Glaseri after the pharmaceutical chemist Christopher Glaser who prepared it and used medicinally.[5][6]
Known as arcanum duplicatum ("double secret") or panacea duplicata in pre-modern medicine, it was prepared from the residue (caput mortuum) left over from the production of aqua fortis, by dissolving the residue in hot water, filtering, and evaporating it to a cuticle. It was then left to crystallise. It was used as a diuretic and sudorific.[7]
According to Chambers's Cyclopedia, the recipe was purchased for five hundred thalers by Charles Frederick, Duke of Holstein-Gottorp. Schroder, the duke's physician, wrote wonders of its great uses in hypochondriacal cases, continued and intermitting fevers, stone, scurvy, etc.[7]
Natural resources
The mineral form of potassium sulfate, arcanite, is relatively rare. Natural resources of potassium sulfate are minerals abundant in the Stassfurt salt. These are cocrystallizations of potassium sulfate and sulfates of magnesium calcium and sodium.
Relevant minerals are:
- Kainite, MgSO4·KCl·H2O
- Schönite (now known as picromerite), K2SO4·MgSO4·6H2O
- Leonite, K2SO4·MgSO4·4H2O
- Langbeinite, K2Mg2(SO4)3
- Glaserite (now known as aphthitalite), K3Na(SO4)2
- Polyhalite, K2SO4·MgSO4·2CaSO4·2H2O
The potassium sulfate can be separated from some of these minerals, like kainite, because the corresponding salt is less soluble in water.
Kieserite, MgSO4·H2O, can be combined with a solution of potassium chloride to produce potassium sulfate.
Production
Approximately 1.5 million tons were produced in 1985, typically by the reaction of potassium chloride with sulfuric acid, analogous to the Leblanc process. Potassium sulfate is produced according to the following reaction, which is conducted in so-called Mannheim furnaces:[8]
- 2 KCl + H2SO4 → 2 HCl + K2SO4
The Hargreaves process uses sulfur dioxide, oxygen and water and potassium chloride as the starting materials to produce potassium sulfate. Hydrochloric acid evaporates. SO2 is produced through the burning of sulfur.
Structure and properties
Two crystalline forms are known. Orthorhombic β-K2SO4 is the common form, but it converts to α-K2SO4 above 583 °C.[8] These structures are complex, although the sulfate adopts the typical tetrahedral geometry.[9]
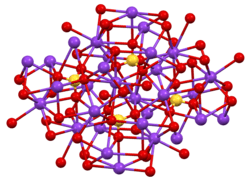 Structure of β-K2SO4.
Structure of β-K2SO4. Coordination sphere of one of two types of K+ site.
Coordination sphere of one of two types of K+ site.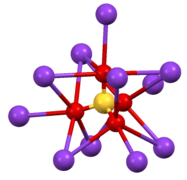 SO4 environment in β-K2SO4.
SO4 environment in β-K2SO4.
It does not form a hydrate, unlike sodium sulfate. The salt crystallize as double six-sided pyramids, classified as rhombic. They are transparent, very hard and have a bitter, salty taste. The salt is soluble in water, but insoluble in solutions of potassium hydroxide (sp. gr. 1.35), or in absolute ethanol.
Uses
The dominant use of potassium sulfate is as a fertilizer. K2SO4 does not contain chloride, which can be harmful to some crops. Potassium sulfate is preferred for these crops, which include tobacco and some fruits and vegetables. Crops that are less sensitive may still require potassium sulfate for optimal growth if the soil accumulates chloride from irrigation water.[10]
The crude salt is also used occasionally in the manufacture of glass. Potassium sulfate is also used as a flash reducer in artillery propellant charges. It reduces muzzle flash, flareback and blast overpressure.
It is sometimes used as an alternative blast media similar to soda in soda blasting as it is harder and similarly water-soluble.[11]
Sometimes, when put over a fire, potassium sulfate can make it purple.
Reactions
Acidification
Potassium hydrogen sulfate (also known as potassium bisulfate), KHSO4, is readily produced by reacting K2SO4 with sulfuric acid. It forms rhombic pyramids, which melt at 197 °C (387 °F). It dissolves in three parts of water at 0 °C (32 °F). The solution behaves much as if its two congeners, K2SO4 and H2SO4, were present side by side of each other uncombined; an excess of ethanol the precipitates normal sulfate (with little bisulfate) with excess acid remaining.
The behavior of the fused dry salt is similar when heated to several hundred degrees; it acts on silicates, titanates, etc., the same way as sulfuric acid that is heated beyond its natural boiling point does. Hence it is frequently used in analytical chemistry as a disintegrating agent. For information about other salts that contain sulfate, see sulfate.
Reduction
At high temperatures, it is reduced to potassium sulfide by the action of carbon monoxide.
References
- ↑ Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8.
- ↑ Windholtz, M (Ed.) & Budavari, S (Ed.), 1983. The Merck Index, Rahway: Merck & Co.
- ↑ Chambers, Michael. "ChemIDplus - 7778-80-5 - OTYBMLCTZGSZBG-UHFFFAOYSA-L - Potassium sulfate [USAN:JAN] - Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.sis.nlm.nih.gov.
- ↑ Thomas, Sunil (2017). "Potassium sulfate forms a spiral structure when dissolved in solution". Russian J Phys Chem B. 11: 195–198. doi:10.1134/S1990793117010328.
- ↑ De Milt, Clara (1942). "Christopher Glaser". Journal of Chemical Education. 19 (2): 53. doi:10.1021/ed019p53.
- ↑ Klooster, van (1959). "Three centuries of Rochelle salt". Journal of Chemical Education. 36 (7): 314. doi:10.1021/ed036p314.
- 1 2

- 1 2 H. Schultz, G. Bauer, E. Schachl, F. Hagedorn, P. Schmittinger "Potassium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a22_039
- ↑ Gaultier, M.; Pannetier, G. "Structure cristalline de la forme 'basse temperature' du sulfate de potassium K2SO4-beta" (Crystal structure of the "low temperature" β-form of potassium sulfate) Bulletin de la Societe Chimique de France 1968, vol. 1, pp. 105-12.
- ↑ organization, United Nations industrial development, UNIDO, International Fertilizer Development Center, IFDC (1998). Fertilizer manual (3rd ed.). Dordrecht: Kluwer academic publ. p. 615. ISBN 0-7923-5032-4.
- ↑ "Super K (Potassium Sulphate)". Retrieved 7 December 2014.