History of the periodic table
The periodic table is an arrangement of the chemical elements and are organized on the basis of their atomic numbers, electron configurations and recurring chemical properties. Elements are presented in order of increasing atomic number. The standard form of the table consists of a grid of elements with rows called periods and columns called groups.
The history of the periodic table reflects over a century of growth in the understanding of chemical properties. The most important event in its history occurred in 1869, when the table was published by Dmitri Mendeleev,[2] who built upon earlier discoveries by scientists such as Antoine-Laurent de Lavoisier and John Newlands, but who is nevertheless generally given sole credit for its development.
Antiquity to the 18th century
A number of physical elements (such as platinum, mercury, tin and zinc) have been known from antiquity, as they are found in their native form and are relatively simple to mine with primitive tools.[3] Around 330 BCE, the Greek philosopher Aristotle proposed that everything is made up of a mixture of one or more roots, an idea that had originally been suggested by the Sicilian philosopher Empedocles. The four roots, which were later renamed as elements by Plato, were earth, water, air and fire. Similar ideas about these four elements also existed in other ancient traditions, such as Indian philosophy. While Aristotle and Plato understood the concept of an element, their ideas did nothing to advance the understanding of the nature of matter.

Hennig Brand
The history of the periodic table is also a history of the discovery of the chemical elements. The first person in history to discover a new element was Hennig Brand, a bankrupt German merchant. Brand tried to discover the Philosopher's Stone — a mythical object that was supposed to turn inexpensive base metals into gold. In 1669 (or later), his experiments with distilled human urine resulted in the production of a glowing white substance, which he called "cold fire" (kaltes Feuer).[4] He kept his discovery secret until 1680, when Robert Boyle rediscovered phosphorus and published his findings. The discovery of phosphorus helped to raise the question of what it meant for a substance to be an element.
In 1661, Boyle defined an element as "those primitive and simple Bodies of which the mixt ones are said to be composed, and into which they are ultimately resolved."[5]
Antoine-Laurent de Lavoisier
Lavoisier's Traité Élémentaire de Chimie (Elementary Treatise of Chemistry), which was written in 1789 and first translated into English by the writer Robert Kerr, is considered to be the first modern textbook about chemistry. Lavoisier defined an element as a substance that cannot be broken down into a simpler substance by a chemical reaction.[6] This simple definition served for a century and lasted until the discovery of subatomic particles. Lavoisier's book contained a list of "simple substances" that Lavoisier believed could not be broken down further, which included oxygen, nitrogen, hydrogen, phosphorus, mercury, zinc and sulfur, which formed the basis for the modern list of elements. Lavoisier's list also included 'light' and 'caloric', which at the time were believed to be material substances. He classified these substances into metals and non metals. While many leading chemists refused to believe Lavoisier's new revelations, the Elementary Treatise was written well enough to convince the younger generation. However, Lavoisier's descriptions of his elements lack completeness, as he only classified them as metals and non-metals.
19th century
William Prout
In 1815, the English physician and chemist William Prout noticed that atomic weights seemed to be multiples of that of hydrogen.[7]
Johann Wolfgang Döbereiner
In 1817, Johann Wolfgang Döbereiner, a chemist, began to formulate one of the earliest attempts to classify the elements.[8] In 1829, he found that he could form some of the elements into groups of three, with the members of each group having related properties. He termed these groups triads.[9]
Definition of Triad law:-"Chemically analogous elements arranged in increasing order of their atomic weights formed well marked groups of three called Triads in which the atomic weight of the middle element was found to be generally the arithmetic mean of the atomic weight of the other two elements in the triad.
Alexandre-Emile Béguyer de Chancourtois
Alexandre-Emile Béguyer de Chancourtois, a French geologist, was the first person to notice the periodicity of the elements — similar elements occurring at regular intervals when they are ordered by their atomic weights. In 1862 he devised an early form of periodic table, which he named Vis tellurique (the 'telluric helix'), after the element tellurium, which fell near the center of his diagram.[10][11] With the elements arranged in a spiral on a cylinder by order of increasing atomic weight, de Chancourtois saw that elements with similar properties lined up vertically. His 1863 publication included a chart (which contained ions and compounds,[12] in addition to elements), but his original paper in the Comptes Rendus de l'Académie des Sciences used geological rather than chemical terms and did not include a diagram. As a result, de Chancourtois' ideas received little attention until after the work of Dmitri Mendeleev had been publicised.[13]
John Newlands
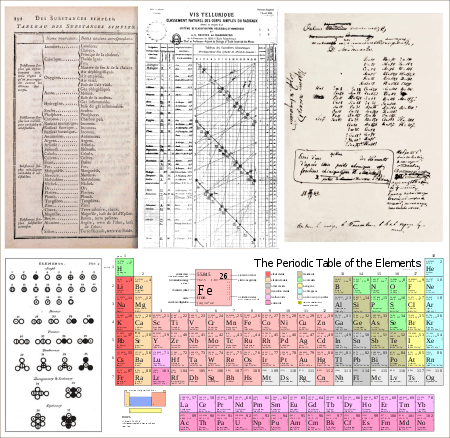
In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.[14][15][16][11]
Newlands noted that many pairs of similar elements existed, which differed by some multiple of eight in mass number, and was the first to assign them an atomic number.[1] When his 'law of octaves' was printed in Chemistry News, likening this periodicity of eights to the musical scale, it was ridiculed by some of his contemporaries. His lecture to the Chemistry Society on 1 March 1866 was not published, the Society defending their decision by saying that such 'theoretical' topics might be controversial.[17]
The importance of Newlands' analysis was eventually recognised by the Chemistry Society with a Gold Medal five years after they recognised Mendeleev's work. It was not until the following century, with Gilbert N. Lewis's valence bond theory (1916) and Irving Langmuir's octet theory of chemical bonding (1919), that the importance of the periodicity of eight would be accepted.[18][19][20] The Royal Chemistry Society acknowledged Newlands' contribution to science in 2008, when they put a Blue Plaque on the house where he was born, which described him as the "discoverer of the Periodic Law for the chemical elements".[1]
He contributed the word 'periodic' in chemistry.
Dmitri Mendeleev
.svg.png)
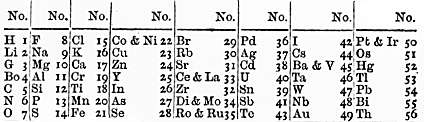
The Russian chemist Dmitri Mendeleev was the first scientist to make a periodic table similar to the one used today. Mendeleev arranged the elements by atomic mass, corresponding to relative molar mass. It is sometimes said that he played 'chemical solitaire' on long train journeys, using cards with various facts about the known elements.[21] On March 6, 1869, Mendeleev gave a formal presentation, The Dependence Between the Properties of the Atomic Weights of the Elements, to the Russian Chemical Society. In 1869, the table was published in an obscure Russian journal and then republished in a German journal, Zeitschrift für Chemie.[22][23] In it, Mendeleev stated that:
- The elements, if arranged according to their atomic mass, exhibit an apparent periodicity of properties.
- Elements which are similar as regards to their chemical properties have atomic weights which are either of nearly the same value (e.g., Pt, Ir, Os) or which increase regularly (e.g., K, Rb, Cs).
- The arrangement of the elements, or of groups of elements in the order of their atomic masses, corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F.
- The elements which are the most widely diffused have small atomic weights.
- The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule determines the character of a compound body.
- We must expect the discovery of many yet unknown elements – for example, elements analogous to aluminium and silicon – whose atomic weight would be between 65 and 75.
- The atomic weight of an element may sometimes be amended by a knowledge of those of its contiguous elements. Thus the atomic weight of tellurium must lie between 123 and 126, and cannot be 128.
- Certain characteristic properties of elements can be foretold from their atomic masses.
Scientific benefits of Mendeleev's table
- It enabled Mendeleev to predict the discovery of new elements and left spaces for them, namely eka-silicon (germanium, discovered in 1885), eka-aluminium (gallium, 1875), and eka-boron (scandium, 1879).[11] Thus, there was no disturbance in the periodic table.
- It could be used by Mendeleev to point out that some of the atomic weights being used at the time were incorrect.
- It provided for variance from atomic weight order.
Shortcomings of Mendeleev's table
- The table was not able to predict the existence of the noble gases, but did, however, leave spaces for yet to be discovered elements to slot into. Time proved this audacious calculation correct. However, when this entire family of elements was discovered, William Ramsay was able to add them to the table as Group 0, without the basic concept of the periodic table being disturbed.
- A single position could not be assigned to hydrogen, which could be placed either in the alkali metals group, the halogens group or separately above the table between boron and carbon.[24]
- The lanthanides were difficult to fit into the table.[11]
- Polonium and radium, discovered by Marie Curie in 1898, also were difficult to fit into the table.[11]
Lothar Meyer
Unknown to Mendeleev, a German chemist, Lothar Meyer, was also working on a periodic table.[11] Although his work was published in 1864, and was done independently of Mendeleev, few historians regard him as an equal co-creator of the periodic table. Meyer's table only included twenty-eight elements, which were not classified by atomic weight, but by valence, and he never reached the idea of predicting new elements and correcting atomic weights. A few months after Mendeleev published his periodic table of the known elements, predicted new elements to help complete his table and corrected the atomic weights of some of the elements, Meyer published a virtually identical periodic table.
Meyer and Mendeleev are considered by some historians of science to be the co-creators of the periodic table, but Mendeleev's accurate prediction of the qualities of undiscovered elements enables him to have the larger share of the credit.
William Odling
In 1864, the English chemist William Odling also drew up a table that was remarkably similar to the table produced by Mendeleev.[25] Odling overcame the tellurium-iodine problem and even managed to get thallium, lead, mercury and platinum into the right groups, which is something that Mendeleev failed to do at his first attempt. Odling failed to achieve recognition, however, since it is suspected that he, as Secretary of the Chemical Society of London, was instrumental in discrediting Newlands' earlier work on the periodic table.[17]
20th century
Frederick Soddy
By 1912 almost 50 different radioactive elements had been found, too many for the periodic table. Frederick Soddy in 1913 found that although they emitted different radiation, many elements were alike in their chemical characteristics so shared the same place on the table.[26] They became known as isotopes, from the Greek eisos topos ("same place").[11][27]
Henry Moseley
In 1914, a year before he was killed in action at Gallipoli, the English physicist Henry Moseley found a relationship between the X-ray wavelength of an element and its atomic number.[28] He was then able to re-sequence the periodic table by nuclear charge, rather than by atomic weight. Before this discovery, atomic numbers were sequential numbers based on an element's atomic weight. Moseley's discovery showed that atomic numbers were in fact based upon experimental measurements.
Using information about their X-ray wavelengths, Moseley placed argon (with an atomic number Z=18) before potassium (Z=19), despite the fact that argon's atomic weight of 39.9 is greater than the atomic weight of potassium (39.1). The new order was in agreement with the chemical properties of these elements, since argon is a noble gas and potassium is an alkali metal. Similarly, Moseley placed cobalt before nickel and was able to explain that tellurium occurs before iodine, without revising the experimental atomic weight of tellurium, as had been proposed by Mendeleev.
Moseley's research showed that there were gaps in the periodic table at atomic numbers 43 and 61, which are now known to be occupied by technetium and promethium respectively.
Glenn T. Seaborg
During his Manhattan Project research in 1943, Glenn T. Seaborg experienced unexpected difficulties in isolating the elements americium and curium. Seaborg wondered if these elements belonged to a different series, which would explain why their chemical properties were different from what was expected. In 1945, against the advice of colleagues, he proposed a significant change to Mendeleev's table: the actinide series.
Seaborg's actinide concept of heavy element electronic structure, predicting that the actinides form a transition series analogous to the rare earth series of lanthanide elements, is now well accepted and included in the periodic table. The actinide series is the second row of the f-block (5f series). In both the actinide and lanthanide series, an inner electron shell is being filled. The actinide series comprises the elements from actinium to lawrencium. Seaborg's subsequent elaborations of the actinide concept theorized a series of superheavy elements in a transactinide series comprising elements from 104 to 121 and a superactinide series of elements from 122 to 153.
See also
References
- 1 2 3 John Newlands, Chemistry Review, November 2003, pp15-16
- ↑ IUPAC article on periodic table Archived 2008-02-13 at the Wayback Machine.
- ↑ Scerri, E. R. (2006). The Periodic Table: Its Story ad Its Significance; New York City, New York; Oxford University Press.
- ↑ Weeks, Mary (1956). Discovery of the Elements (6th ed.). Easton, Pennsylvania, USA: Journal of Chemical Education. p. 122.
- ↑ Boyle, Robert (1661). The Skeptical Chymist. London, England: J. Crooke. p. 16.
- ↑ Lavoisier with Robert Kerr, trans. (1790) Elements of Chemistry. Edinburgh, Scotland: William Creech. From p. xxiv: "I shall therefore only add upon this subject, that if, by the term elements, we mean to express those simple and indivisible atoms of which matter is composed, it is extremely probable we know nothing at all about them; but, if we apply the term elements, or principles of bodies, to express our idea of the last point which analysis is capable of reaching, we must admit, as elements, all substances into which we are capable, by any means, to reduce bodies by decomposition. Not that we are entitled to affirm, that these substances we consider as simple may not be compounded of two, or even of a greater number of principles; but, since these principles cannot be separated, or rather since we have not hitherto discovered means of separating them, they act with regard to us as simple substances, and we ought never to suppose them compounded until experiment and observation has proved them to be so."
- ↑ See:
- Prout, William (November 1815). "On the relation between the specific gravities of bodies in their gaseous state and the weights of their atoms". Annals of Philosophy. 6: 321–330.
- Prout, William (February 1816). "Correction of a mistake in the essay on the relation between the specific gravities of bodies in their gaseous state and the weights of their atoms". Annals of Philosophy. 7: 111–113.
- ↑ Wurzer, Ferdinand (1817). "Auszug eines Briefes vom Hofrath Wurzer, Prof. der Chemie zu Marburg" [Excerpt of a letter from Court Advisor Wurzer, Professor of Chemistry at Marburg]. Annalen der Physik (in German). 56: 331–334. Here, Döbereiner found that strontium's properties were intermediate to those of calcium and barium.
- ↑ Döbereiner, J. W. (1829). "Versuch zu einer Gruppirung der elementaren Stoffe nach ihrer Analogie" [An attempt to group elementary substances according to their analogies]. Annalen der Physik und Chemie. 2nd series (in German). 15: 301–307. For an English translation of this article, see: Johann Wolfgang Döbereiner: "An Attempt to Group Elementary Substances according to Their Analogies" (Lemoyne College (Syracuse, New York, USA))
- ↑ Beguyer de Chancourtois (1862). "Tableau du classement naturel des corps simples, dit vis tellurique" [Table of the natural classification of elements, called the "telluric helix"]. Comptes Rendus de l'Académie des Sciences (in French). 55: 600–601.
- 1 2 3 4 5 6 7 Ley, Willy (October 1966). "The Delayed Discovery". For Your Information. Galaxy Science Fiction. pp. 116–127.
- ↑ Chancourtois, Alexandre-Émile Béguyer de (1863). Vis tellurique. Classement des corps simples ou radicaux, obtenu au moyen d'un système de classification hélicoïdal et numérique (in French). Paris, France: Mallet-Bachelier. 21 pages.
- ↑ Annales des Mines history page.
- ↑ See:
- Newlands, John A. R. (7 February 1863). "On relations among the equivalents". The Chemical News. 7: 70–72.
- Newlands, John A. R. (30 July 1864). "Relations between equivalents". The Chemical News. 10: 59–60.
- Newlands, John A. R. (20 August 1864). "On relations among the equivalents". The Chemical News. 10: 94–95.
- Newlands, John A. R. (18 August 1865). "On the law of octaves". The Chemical News. 12: 83.
- (Editorial staff) (9 March 1866). "Proceedings of Societies: Chemical Society: Thursday, March 1". The Chemical News. 13: 113–114.
- Newlands, John A.R. (1884). On the Discovery of the Periodic Law and on Relations among the Atomic Weights. E. & F.N. Spon: London, England.
- ↑ in a letter published in Chemistry News in February 1863, according to the Notable Names Data Base
- ↑ Newlands on classification of elements
- 1 2 Shaviv, Giora (2012). The Synthesis of the Elements. Berlin, Germany: Springer-Verlag. p. 38. From p. 38: "The reason [for rejecting Newlands' paper, which was] given by Odling, then the president of the Chemical Society, was that they made a rule not to publish theoretical papers, and this on the quite astonishing grounds that such papers lead to a correspondence of controversial character."
- ↑ Lewis, Gilbert N. (1916). "The atom and the molecule". Journal of the American Chemical Society. 38: 762–785.
- ↑ Langmuir, Irving (1919). "The structure of atoms and the octet theory of valence". Proceedings of the National Academy of Sciences of the United States of America. 5: 252–259. Bibcode:1919PNAS....5..252L. doi:10.1073/pnas.5.7.252. PMC 1091587.
- ↑ Langmuir, Irving (1919). "The arrangement of electrons in atoms and molecules". Journal of the American Chemical Society. 41 (6): 868–934.
- ↑ Physical Science, Holt Rinehart & Winston (January 2004), page 302 ISBN 0-03-073168-2
- ↑ Менделеев, Д. (1869). "Соотношение свойств с атомным весом элементов" [Relationship of elements' properties to their atomic weights]. Журнал Русского Химического Общества (Journal of the Russian Chemical Society) (in Russian). 1: 60–77.
- ↑ Mendeleev, Dmitri (1869). "Ueber die Beziehungen der Eigenschaften zu den Atomgewichten der Elemente" [On the relations of elements' properties to their atomic weights]. Zeitschrift für Chemie. 12: 405–406.
- ↑ "Reed Magazine: The Alumni Association: Around the World in 80 Seconds". reed.edu. Retrieved 6 March 2017.
- ↑ See:
- Odling, William (June 1857). "On the natural groupings of the elements. Part 1". Philosophical Magazine. 4th series. 13 (88): 423–440.
- Odling, William (1857). "On the natural groupings of the elements. Part 2". Philosophical Magazine. 4th series. 13 (89): 480–497.
- Odling, William (1864). "On the hexatomicity of ferricum and aluminium". Philosophical Magazine. 4th series. 27 (180): 115–119.
- Odling, William (1864). "On the proportional numbers of the elements". Quarterly Journal of Science. 1: 642–648.
- ↑ See:
- Soddy, Frederick (1913). "Radioactivity". Annual Reports on the Progress of Chemistry. 10: 262–288.
- Soddy, Frederick (28 February 1913). "The radio-elements and the periodic law". The Chemical News. 107 (2779): 97–99.
- ↑ Soddy first used the word "isotope" in: Soddy, Frederick (4 December 1913). "Intra-atomic charge". Nature. 92 (2301): 399–400. Bibcode:1913Natur..92..399S. doi:10.1038/092399c0. See p. 400.
- ↑ Moseley, H.G.J. (1914). "The high-frequency spectra of the elements". Philosophical Magazine. 6th series. 27: 703–713.
External links
- Development of the periodic table (part of a collection of pages that explores the periodic table and the elements) by the Royal Society of Chemistry
- Dr. Eric Scerri's web page, which has links to interviews, lectures and articles on various aspects of the periodic system, including the history of the periodic table.
- The Internet Database of Periodic Tables – a large collection of periodic tables and periodic system formulations.
- History of Mendeleev periodic table of elements as a data visualization at CrossValidated Stack Exchange


