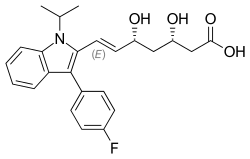
Fluvastatin
 | |
| Clinical data | |
|---|---|
| Trade names | Lescol, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a694010 |
| Pregnancy category | |
| Routes of administration | By mouth (capsules, tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 24–30%[1][2] |
| Protein binding | >98%[2] |
| Metabolism | Hepatic: CYP2C9 (75%), CYP3A4 (20%), CYP2C8 (5%)[2][3] |
| Elimination half-life | 1–3 hours (capsule), 9 hours (XR formulations)[2][3] |
| Excretion | Faeces (95%), urine (5%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.224.327 |
| Chemical and physical data | |
| Formula | C24H26FNO4 |
| Molar mass | 411.466 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fluvastatin (INN,[4] trade names Lescol, Canef, Vastin) is a member of the statin drug class, used to treat hypercholesterolemia and to prevent cardiovascular disease.
Adverse effects
Adverse effects are comparable to other statins. Common are nausea, indigestion, insomnia and headache. Myalgia (muscle pain), and rarely rhabdomyolysis, characteristic side effects for statins, can also occur.[5]
Interactions
Contrary to lovastatin, simvastatin and atorvastatin, fluvastatin has no relevant interactions with drugs that inhibit the liver enzyme CYP3A4, and a generally lower potential for interactions than most other statins. Fluconazole, a potent inhibitor of CYP2C9, does increase fluvastatin levels.[5]
Pharmacology
Mechanism of action
Fluvastatin works by blocking the liver enzyme HMG-CoA reductase, which facilitates an important step in cholesterol synthesis.[1]
Pharmacodynamics
In a Cochrane systematic review the dose-related magnitudes of fluvastatin on blood lipids was determined. Over the dose range of 10 to 80 mg/day total cholesterol was reduced by 10.7% to 24.9%, LDL cholesterol by 15.2% to 34.9%, and triglycerides by 3% to 17.5%.[6]
Pharmacokinetics
The drug is quickly and almost completely (98%) absorbed from the gut. Food intake slows down absorption, but does not decrease it. Due to its first-pass effect, bioavailability is lower: about 24–30%[2][1] according to different sources. Over 98% of the substance is bound to plasma proteins.[1]
Several cytochrome P450 enzymes (mainly CYP2C9, but also CYP3A4 and CYP2C8)[7] are involved in the metabolism of fluvastatin, which makes is less liable to interactions than most other statins. The main metabolite is inactive and is called "N-desisopropyl propionic acid" in the literature.[1][5]
93–95% of the drug is excreted via the feces, less than 2% of which in form of the original substance.[1]
Research
Fluvastatin has also been shown to exhibit antiviral activity against hepatitis C virus in a study with 31 patients. This effect has been described as modest, variable, and often short-lived, by the authors.[8] Data from the Cholesterol Treatment Trialists’ (CTT) publication[9] was used to determine the effects of fluvastatin, atorvastatin and rosuvastatin on LDL cholesterol lowering and reduction of myocardial infarction. In two RCTs an average dose of 72 mg/day fluvastatin reduced LDL cholesterol by 31.9%, and reduced myocardial infarction, relative risk, 0.68 (95% CI 0.55 to 0.85) as compared to placebo. In five RCTs a mean atorvastatin dose of 26 mg/day reduced LDL cholesterol by 44.0% and reduced myocardial infarction, relative risk, 0.67 (95% CI 0.58 to 0.77) as compared to placebo. In four RCTs a mean rosuvastatin dose of 16 mg/day reduced LDL cholesterol by 48.8% and reduced myocardial infarction, relative risk, 0.82 (95% CI 0.73 to 0.93) as compared to placebo. Thus despite reducing LDL cholesterol by a much lesser amount with fluvastatin than atorvastatin and rosuvastatin, fluvastatin reduced myocardial infarction similarly to atorvastatin and to a greater degree than rosuvastatin.[6]
References
- 1 2 3 4 5 6 Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- 1 2 3 4 5 6 Neuvonen, PJ; Backman, JT; Niemi, M (2008). "Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin". Clinical Pharmacokinetics. 47 (7): 463–74. doi:10.2165/00003088-200847070-00003. PMID 18563955.
- 1 2 "Lescol, Lescol XR (fluvastatin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 18 March 2014.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names (Rec. INN): List 30" (PDF). World Health Organization. 1990. Retrieved 29 November 2016.
- 1 2 3 Dinnendahl, V, Fricke, U, eds. (2012). "Arzneistoff-Profile" (in German). 2 (26 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- 1 2 Adams, Stephen P.; Sekhon, Sarpreet S.; Tsang, Michael; Wright, James M. (2018-03-06). Fluvastatin for lowering lipids. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. doi:10.1002/14651858.cd012282.pub2.
- ↑ Lescol Monograph on Drugs.com.
- ↑ Bader T, Fazili J, Madhoun M, et al. (April 2008). "Fluvastatin Inhibits Hepatitis C Replication in Humans". Am. J. Gastroenterol. 103 (6): 1383–9. doi:10.1111/j.1572-0241.2008.01876.x. PMID 18410471.
- ↑ Cholesterol Treatment Trialists’ (CTT). (2005). "Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins". Lancet. 366 (9493): 1267–1278. doi:10.1016/s0140-6736(05)67394-1. ISSN 0140-6736.