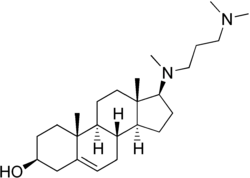
Azacosterol
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms |
20,25-Diazacholesterol; 20,25-Azacholesterol; Azasterol; Diazasterol; SC-12937; DAC; IMD-760; 17β-(3-(Dimethylamino)propyl)methyl- amino)androst-5-en-3β-ol |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H44N2O |
| Molar mass | 388.63 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Azacosterol (INN), or azacosterol hydrochloride (USAN) (brand name Ornitrol), also known as 20,25-diazacholesterol, is a cholesterol-lowering drug (hypocholesteremic) which was marketed previously but has since been discontinued.[1][2][3] It is also an avian chemosterilant used to control the pest pigeon population via inducing sterility.[4] The drug is a sterol and derivative of cholesterol in which two carbon atoms have been replaced with nitrogen atoms.[5]
Azacosterol acts as an inhibitor of 24-dehydrocholesterol reductase (24-DHCR), preventing the formation of cholesterol from desmosterol.[4][6] Although it primarily acts to inhibit 24-DHCR, the drug also inhibits other steps in cholesterol biosynthesis.[6] The anti-fertility effects of the drug in birds are mediated by inhibition of steroid hormone production, steroid hormones being synthesized from cholesterol.[4] Due to prevention of the metabolism of desmosterol, the drug causes it to accumulate, in turn producing side effects such as hyperkeratosis, particularly of the palms and soles.[6]
See also
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 110–. ISBN 978-1-4757-2085-3.
- ↑ I.K. Morton; Judith M. Hall (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 43–. ISBN 978-0-7514-0499-9.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 463–. ISBN 978-0-8155-1856-3.
- 1 2 3 Progress in Medicinal Chemistry. Elsevier. 1 January 1979. pp. 79–. ISBN 978-0-08-086264-4.
- ↑ Counsell, R. E.; Klimstra, P. D.; Ranney, R. E. (1962). "Hypocholesterolemic Agents. III.1N-Methyl-N-(dialkylamino)alkyl-17β-aminoandrost-5-en-3β-ol Derivatives". Journal of Medicinal and Pharmaceutical Chemistry. 5 (6): 1224. doi:10.1021/jm01241a014. PMID 14056455.
- 1 2 3 Peter M. Elias (21 January 2016). Advances in Lipid Research: Skin Lipids. Elsevier. pp. 218–220. ISBN 978-1-4832-1545-7.