Canis
| Canis | |
|---|---|
 | |
| Gray wolf (top), coyote and African golden wolf (top middle), Ethiopian wolf and golden jackal (bottom middle), black-backed jackal and side-striped jackal (bottom) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Family: | Canidae |
| Subfamily: | Caninae |
| Tribe: | Canini |
| Genus: | Canis Linnaeus, 1758[1] |
| Extant species | |
|
1 C. lupus also includes domestic dogs, C. l. familiaris, and dingos, C. l. dingo | |
Canis is a genus of the Canidae containing multiple extant species, such as wolves, coyotes, jackals, dingoes, and dogs. Species of this genus are distinguished by their moderate to large size, their massive, well-developed skulls and dentition, long legs, and comparatively short ears and tails.[2]
Etymology
The generic name Canis means "dog" in Latin. The term "canine" comes from the adjective form, caninus ("of the dog"), from which the term canine tooth is also derived.[3] The canine family has prominent canine teeth, used for killing their prey. The word canis is cognate to the Greek word kūon (Greek: Κύων), which means "dog", as well as (less transparently) English hound.
Terminology
- Immature dogs (that is, animals that are incapable of reproduction) are referred to as pups or puppies,[4] whereas the young of other canines are known as cubs.
- A group of puppies from the same gestation period is referred to as a litter.[5]
Taxonomy
Canini
The tribe Canini[6] (Fischer de Waldheim, 1817) is the sister group to the true foxes (Vulpes), and is represented today by two sub-tribes: Canina, which includes the genus Canis[7] (wolves, jackals, the coyote, and the domestic dog), as well as the dhole and the African wild dog; and Cerdocyonina,[8] which includes the so-called foxes of South America.[9]:p55 The critical features that mark the Canini as a monophyletic group include: the consistent enlargement of the frontal sinus, often accompanied by the correlated loss of the depression in the dorsal surface of the postorbital process; the posterior expansion of the paroccipital process; the enlargement of the mastoid process; and the lack of lateral flare of the orbital border of the zygoma.[9]:p77
Canis
The genus Canis (Carl Linnaeus, 1758) was published in the 10th edition of Systema Naturae[1] and included the dog-like carnivores: the domestic dog, wolves, coyotes and jackals. All species within Canis are phylogenetically closely related with 78 chromosomes and can potentially interbreed.[10] In 1926, the International Commission on Zoological Nomenclature (ICZN) in Opinion 91 included Genus Canis on its Official Lists and Indexes of Names in Zoology.[11] In 1955, the ICZN's Direction 22 added Canis familiaris as the type specimen for genus Canis to the official list.[12]
Evolution
The fossil record shows that feliforms and caniforms emerged within the clade Carnivoramorpha 43 million YBP.[13] The caniforms included the fox-like genus Leptocyon whose various species existed from 24 million YBP before branching 11.9 million YBP into Vulpes (foxes) and Canini (canines). The jackal-sized Eucyon existed in North America from 10 million YBP and by the Early Pliocene about 6-5 million YBP the coyote-like Eucyon davisi[14] invaded Eurasia. In North America it gave rise to early Canis which first appeared in the Miocene (6 million YBP) in south-western USA and Mexico. By 5 million YBP the larger Canis lepophagus appeared in the same region.[15]:p58

The canids that had emigrated from North America to Eurasia – Eucyon, Vulpes, and Nyctereutes – were small to medium-sized predators during the Late Miocene and Early Pliocene but they were not the top predators. The position of the canids would change with the arrival of Canis to become a dominant predator across the Holarctic. The wolf-sized C. chihilensis appeared in northern China in the Mid-Pliocene around 4-3 million YBP. This was followed by an explosion of Canis evolution across Eurasia in the Early Pleistocene around 1.8 million YBP in what is commonly referred to as the wolf event. It is associated with the formation of the mammoth steppe and continental glaciation. Canis spread to Europe in the forms of C. arnensis, C. etruscus, and C. falconeri.[15]:p148 One study found that the diversity of the Canis group decreased by the end of the Early Pleistocene to the Middle Pleistocene and was limited in Eurasia to the small wolves of the Canis mosbachensis–Canis variabilis group and the large hypercarnivorous Canis (Xenocyon) lycaonoides.[16]
- See further: Evolution of the canids
Dentition and biteforce
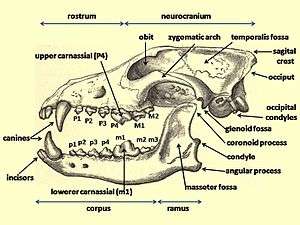

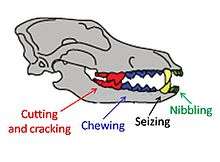
Dentition relates to the arrangement of teeth in the mouth, with the dental notation for the upper-jaw teeth using the upper-case letters I to denote incisors, C for canines, P for premolars, and M for molars, and the lower-case letters i, c, p and m to denote the mandible teeth. Teeth are numbered using one side of the mouth and from the front of the mouth to the back. In carnivores, the upper premolar P4 and the lower molar m1 form the carnassials that are used together in a scissor-like action to shear the muscle and tendon of prey.[15]:74
Canids use their premolars for cutting and crushing except for the upper fourth premolar P4 (the upper carnassial) that is only used for cutting. They use their molars for grinding except for the lower first molar m1 (the lower carnassial) that has evolved for both cutting and grinding depending on the candid's dietary adaptation. On the lower carnassial the trigonid is used for slicing and the talonid is used for grinding. The ratio between the trigonid and the talonid indicates a carnivore's dietary habits, with a larger trigonid indicating a hypercarnivore and a larger talonid indicating a more omnivorous diet.[17][18] Because of its low variability, the length of the lower carnassial is used to provide an estimate of a carnivore's body size.[17]
A study of the estimated bite force at the canine teeth of a large sample of living and fossil mammalian predators, when adjusted for their body mass, found that for placental mammals the bite force at the canines (in Newtons/kilogram of body weight) was greatest in the extinct dire wolf (163), followed among the modern canids by the four hypercarnivores that often prey on animals larger than themselves: the African hunting dog (142), the gray wolf (136), the dhole (112), and the dingo (108). The bite force at the carnassials showed a similar trend to the canines. A predator's largest prey size is strongly influenced by its biomechanical limits.[19]
Behaviour
The difference between the male and female of a species apart from their sex organs is called sexual dimorphism. An example is the male lion, which compared with the female lion possesses a mane, larger size, larger upper canines, and operates in a group of no more than two or three. There is little variance among male and female canids. Canids tend to live as monogamous pairs, and wolves, dholes, and jackals also along with their offspring. Wolves may live in extended family groups. To take prey larger than themselves, the African wild dog, the dhole, and the gray wolf depend on their jaws as they cannot use their forelimbs to grapple with prey. They work together as a pack consisting of an alpha pair and their offspring from the current and previous years.[20] Social mammal predators prey on herbivores with a body mass similar to that of the combined mass of the predator pack.[21][22] The gray wolf specializes in preying on the vulnerable individuals of large prey,[23] and a pack of timber wolves can bring down a 500 kg (1,100 lb) moose.[24][25]
Mating Behaviour
The genus Canis contains many different species and has a wide range of different mating systems that varies depending on the type of canine and the species.[26] In a study done in 2017 it was found that in some species of canids females use their sexual status to gain food resources. The study looked at wolves and dogs. Wolves are typically monogamous and form pair-bonds; whereas dogs are promiscuous when free-range and mate with multiple individuals. The study found that in both species females tried to gain access to food more and were more successful in monopolize a food resource when in heat. Outside of the breeding season their efforts were not as persistent or successful. This shows that the food-for-sex hypothesis likely plays a role in the food sharing among canids and acts as a direct benefit for the females.
Another study on free-ranging dogs found that social factors played a significant role in the determination of mating pairs. The study, done in 2014, looked at social regulation of reproduction in the dogs.[27] They found that females in heat searched out dominant males and were more likely to mate with a dominant male who appeared to be a quality leader. The females were more likely to reject submissive males. Furthermore, cases of male-male competition were more aggressive in the presence of high ranking females. This suggests that females prefer dominant males and males prefer high ranking females meaning social cues and status play a large role in the determination of mating pairs in dogs.
Canids also show a wide range of parental care and in 2018 a study showed that sexual conflict plays a role in the determination of intersexual parental investment.[28] The studied looked at coyote mating pairs and found that paternal investment was increased to match or near match the maternal investment. The amount of parental care provided by the fathers also was shown to fluctuated depending on the level of care provided by the mother.
Another study on parental investment showed that in free-ranging dogs, mothers modify their energy and time investment into their pups as they age.[29] Due to the high mortality of free-range dogs at a young age a mother's fitness can be drastically reduced. This study found that as the pups aged the mother shifted from high-energy care to lower-energy care so that they can care for their offspring for a longer duration for a reduced energy requirement. By doing this the mothers increasing the likelihood of their pups surviving infancy and reaching adulthood and thereby increase their own fitness.
A study done in 2017 found that aggression between male and female grey wolves varied and changed with age.[30] Males were more likely to chase away rival packs and lone individuals than females and became increasingly aggressive with age. Alternatively, females were found to be less aggressive and constant in their level of aggression throughout their life. This requires further research but suggests that intersexual aggression levels in grey wolves relates to their mating system.
Tooth breakage
Tooth breakage is a frequent result of carnivores' feeding behaviour.[31] Carnivores include both pack hunters and solitary hunters. The solitary hunter depends on a powerful bite at the canine teeth to subdue their prey, and thus exhibits a strong mandibular symphysis. In contrast, a pack hunter, which delivers many shallower bites, has a comparably weaker mandibular symphysis. Thus, researchers can use the strength of the mandibular symphysis in fossil carnivore specimens to determine what kind of hunter it was – a pack hunter or a solitary hunter – and even how it consumed its prey. The mandibles of canids are buttressed behind the carnassial teeth to crack bones with their post-carnassial teeth (molars M2 and M3). A study found that the modern gray wolf and the red wolf (C. rufus) possess greater buttressing than all other extant canids and the extinct dire wolf. This indicates that these are both better adapted for cracking bone than other canids.[32]
A study of nine modern carnivores indicate that one in four adults had suffered tooth breakage and that half of these breakages were of the canine teeth. The highest frequency of breakage occurred in the spotted hyena, which is known to consume all of its prey including the bone. The least breakage occurred in the African wild dog. The gray wolf ranked between these two.[31][33] The eating of bone increases the risk of accidental fracture due to the relatively high, unpredictable stresses that it creates. The most commonly broken teeth are the canines, followed by the premolars, carnassial molars, and incisors. Canines are the teeth most likely to break because of their shape and function, which subjects them to bending stresses that are unpredictable in direction and magnitude.[33] The risk of tooth fracture is also higher when taking and consuming large prey.[33][34]
In comparison to extant gray wolves, the extinct Beringian wolves included many more individuals with moderately to heavily worn teeth and with a significantly greater number of broken teeth. The frequencies of fracture ranged from a minimum of 2% found in the Northern Rocky Mountain wolf (Canis lupus irremotus) up to a maximum of 11% found in Beringian wolves. The distribution of fractures across the tooth row also differs, with Beringian wolves having much higher frequencies of fracture for incisors, carnassials, and molars. A similar pattern was observed in spotted hyenas, suggesting that increased incisor and carnassial fracture reflects habitual bone consumption because bones are gnawed with the incisors and then cracked with the carnassials and molars.[35]
Wolves, dogs, and dingoes
Wolves, dogs, and dingoes are subspecies of Canis lupus. The original referent of the English word wolf, the Eurasian wolf, is called C. l. lupus to distinguish it from other wolf subspecies, such as the Indian wolf (C. l. pallipes), the Arabian wolf (C. l. arabs), or the Tibetan wolf (C. l. filchneri).
Some experts have suggested some subspecies of C. lupus be considered Canis species distinct from C. lupus. These include Central Asia's Himalayan wolf, and the Indian wolf,[36][37] as well as North America's red wolf and eastern wolf.[38]
The dingo (C. l. dingo), from Australasia, and the domestic dog (C. l. familiaris) are also considered subspecies of C. lupus, although they are not commonly referred to or thought of as "wolves".[39]
Coyotes, jackals, and wolves
| Phylogenetic tree of the extant wolf-like canids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phylogenetic relationships between the extant wolf-like clade of canids based on nuclear DNA (15 kilobytes of exon and intron sequence data taken from the cell nucleus),[40][41] except for the Himalayan wolf based on mitochondrial DNA sequences.[41][42][43][44] All species belong to the genus Canis, apart from the dhole (Cuon) and the African wild dog (Lycaon) that are descended from the extinct Canis subgenus named Xenocyon.[15]:p149 Time of divergence in millions of years.[41][45] |
The gray wolf (C. lupus), the Ethiopian wolf (C. simensis), and the African golden wolf (C. anthus) are three of the many Canis species referred to as "wolves"; however, all of the others are now extinct and little is known about them by the general public. One of these, the extinct dire wolf (C. dirus), has gained fame from the thousands of specimens found and displayed at the Rancho La Brea Tar Pits in Los Angeles, California.
Canis species that are too small to attract the word "wolf" are called coyotes in the Americas and jackals elsewhere. Although these may not be more closely related to each other than they are to C. lupus, they are, as fellow Canis species, all more closely related to wolves and domestic dogs than they are to foxes, maned wolves, or other canids which do not belong to the genus Canis. The word "jackal" is applied to three distinct species of this group: the side-striped (C. adustus) and black-backed (C. mesomelas) jackals, found in sub-Saharan Africa, and the golden jackal (C. aureus), found across southwestern and south-central Asia, and the Balkans.
While North America has only one small-sized species, the coyote (C. latrans), it has become very widespread, moving into areas once occupied by wolves. They can be found across much of mainland Canada, in every state of the contiguous United States, all of Mexico except the Yucatán Peninsula, and the Pacific and central areas of Central America, ranging as far as western Panama.
African migration
The first record of genus Canis on the African continent is Canis sp. A from South Turkwel, Kenya dated 3.58–3.2 million years ago.[46] In 2015, a study of mitochondrial genome sequences and whole genome nuclear sequences of African and Eurasian canids indicated that extant wolf-like canids have colonised Africa from Eurasia at least 5 times throughout the Pliocene and Pleistocene, which is consistent with fossil evidence suggesting that much of African canid fauna diversity resulted from the immigration of Eurasian ancestors, likely coincident with Plio-Pleistocene climatic oscillations between arid and humid conditions.[47]:S1 In 2017, the fossil remains of a new Canis species named Canis othmanii was discovered among remains found at Wadi Sarrat, Tunisia from deposits that date 700,000 years ago. This canine shows a morphology more closely associated with canids from Eurasia rather than Africa.[48]
Gallery

.jpg) Eastern wolf (Canis lycaon) (often includes latrans admixture)
Eastern wolf (Canis lycaon) (often includes latrans admixture).jpg) Red wolf (Canis rufus) (includes latrans admixture)
Red wolf (Canis rufus) (includes latrans admixture) Coyote (Canis latrans)
Coyote (Canis latrans) Dire wolf (Canis dirus) (extinct)
Dire wolf (Canis dirus) (extinct) African golden wolf (Canis anthus)
African golden wolf (Canis anthus) Golden jackal (Canis aureus)
Golden jackal (Canis aureus) Ethiopian wolf (Canis simensis)
Ethiopian wolf (Canis simensis) Black-backed jackal (Canis mesomelas)
Black-backed jackal (Canis mesomelas)-_rare_sighting_of_this_nocturnal_animal_..._(13799182833).jpg) Side-striped jackal (Canis adustus)
Side-striped jackal (Canis adustus)
See also
References
- 1 2 Linnæus, Carl (1758). Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I (in Latin) (10th ed.). Holmiæ (Stockholm): Laurentius Salvius. p. 38. Retrieved November 23, 2015.
- ↑ Heptner, V. G.; Naumov, N. P. (1998). Mammals of the Soviet Union Vol.II Part 1a, SIRENIA AND CARNIVORA (Sea Cows, Wolves and Bears). Science Publishers, Inc. USA. pp. 124-129. ISBN 1-886106-81-9.
- ↑ Harper, Douglas. "canine". Online Etymology Dictionary.
- ↑ Puppy in the Oxford English Dictionary
- ↑ Litter in the Oxford English Dictionary (may also refer to young cats)
- ↑ Fossilworks website Canini
- ↑ Fossilworks website Canis
- ↑ Fossilworks website Cerdocyonina
- 1 2 Tedford, R (2009). "Phylogenetic Systematics of the North American Fossil Caninae (Carnivora: Canidae)". Bulletin of the American Museum of Natural History. 325: 1–218. doi:10.1206/574.1.
- ↑ Wayne, R. (1999). "Origin, genetic diversity, and genome structure of the domestic dog". BioEssays. 21 (3): 247–57. doi:10.1002/(SICI)1521-1878(199903)21:3<247::AID-BIES9>3.0.CO;2-Z. PMID 10333734.
- ↑ "Opinions and Declarations Rendered by the International Commission on Zoological Nomenclature - Opinion 91". Smithsonian Miscellaneous Collections. Smithsonian Institution, City of Washington. 73 (4). 1926.
- ↑ Francis Hemming, ed. (1955). "Direction 22". Opinions and Declarations Rendered by the International Commission on Zoological Nomenclature. 1C. Order of the International Trust for Zoological Nomenclature. p. 183.
- ↑ Flynn, John J.; Wesley-Hunt, Gina D. (2005). "Phylogeny of the Carnivora: Basal Relationships Among the Carnivoramorphans, and Assessment of the Position of 'Miacoidea' Relative to Carnivora". Journal of Systematic Palaeontology. 3: 1–28. doi:10.1017/s1477201904001518.
- ↑ Fossilworks website Eucyon davisi
- 1 2 3 4 Wang, Xiaoming; Tedford, Richard H. (2008). Dogs: Their Fossil Relatives and Evolutionary History. Columbia University Press. ISBN 978-0-231-13528-3. OCLC 502410693.
- ↑ Sotnikova, M (2010). "Dispersal of the Canini (Mammalia, Canidae: Caninae) across Eurasia during the Late Miocene to Early Pleistocene". Quaternary International. 212 (2): 86–97. Bibcode:2010QuInt.212...86S. doi:10.1016/j.quaint.2009.06.008.
- 1 2 Sansalone, Gabriele; Bertè, Davide Federico; Maiorino, Leonardo; Pandolfi, Luca (2015). "Evolutionary trends and stasis in carnassial teeth of European Pleistocene wolf Canis lupus (Mammalia, Canidae)". Quaternary Science Reviews. 110: 36. doi:10.1016/j.quascirev.2014.12.009.
- ↑ Cherin, Marco; Bertè, Davide Federico; Sardella, Raffaele; Rook, Lorenzo (2013). "Canis etruscus (Canidae, Mammalia) and its role in the faunal assemblage from Pantalla (Perugia, central Italy): comparison with the Late Villafranchian large carnivore guild of Italy" (PDF). Bollettino della Società Paleontologica Italiana. 52 (1): 11–18.
- ↑ Wroe, S.; McHenry, C.; Thomason, J. (2005). "Bite club: Comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa". Proceedings of the Royal Society B: Biological Sciences. 272 (1563): 619–25. doi:10.1098/rspb.2004.2986. PMC 1564077. PMID 15817436.
- ↑ Van Valkenburgh, Blaire; Sacco, Tyson (2002). "Sexual dimorphism, social behavior, and intrasexual competition in large Pleistocene carnivorans". Journal of Vertebrate Paleontology. 22: 164–169. doi:10.1671/0272-4634(2002)022[0164:SDSBAI]2.0.CO;2.
- ↑ Sorkin, Boris (2008). "A biomechanical constraint on body mass in terrestrial mammalian predators". Lethaia. 41 (4): 333–347. doi:10.1111/j.1502-3931.2007.00091.x.
- ↑ Earle, M. (1987). "A flexible body mass in social carnivores". American Naturalist. 129 (5): 755–760. doi:10.1086/284670.
- ↑ Paquet, P; Carbyn, L. W. (2003). "23-Gray Wolf (Canis Inpus and Allies)". In Feldhamer, George A. Wild Mammal of North America: Biology, Management, and Conservation. Nature. pp. 482–509. ISBN 0-8018-7416-5.
- ↑ Mech, L. David (1966). The Wolves of Isle Royale. Fauna Series 7. Fauna of the National Parks of the United States. p. 76. ISBN 1-4102-0249-6. Retrieved 1 May 2017.
- ↑ Anyonge, William; Roman, Chris (2006). "New body mass estimates for Canis dirus, the extinct Pleistocene dire wolf". Journal of Vertebrate Paleontology. 26: 209–212. doi:10.1671/0272-4634(2006)26[209:NBMEFC]2.0.CO;2.
- ↑ Dale, Rachel; Marshall-Pescini, Sarah; Range, Friederike (2017-06-01). "Do females use their sexual status to gain resource access? Investigating food-for-sex in wolves and dogs". Current Zoology. 63 (3). doi:10.1093/cz/zow111. ISSN 1674-5507.
- ↑ Cafazzo, Simona; Bonanni, Roberto; Valsecchi, Paola; Natoli, Eugenia (2014-06-06). "Social Variables Affecting Mate Preferences, Copulation and Reproductive Outcome in a Pack of Free-Ranging Dogs". PLOS ONE. 9 (6): e98594. doi:10.1371/journal.pone.0098594. ISSN 1932-6203.
- ↑ Schell, Christopher J; Young, Julie K; Lonsdorf, Elizabeth V; Mateo, Jill M; Santymire, Rachel M. "It takes two: Evidence for reduced sexual conflict over parental care in a biparental canid". Journal of Mammalogy. 99 (1): 75–88. doi:10.1093/jmammal/gyx150.
- ↑ Paul, Manabi; Sau, Shubhra; Nandi, Anjan K.; Bhadra, Anindita (2017-01-01). "Clever mothers balance time and effort in parental care: a study on free-ranging dogs". Open Science. 4 (1): 160583. arXiv:1607.01135. Bibcode:2017RSOS....460583P. doi:10.1098/rsos.160583. ISSN 2054-5703.
- ↑ Cassidy, Kira A.; Mech, L. David; MacNulty, Daniel R.; Stahler, Daniel R.; Smith, Douglas W. "Sexually dimorphic aggression indicates male gray wolves specialize in pack defense against conspecific groups". Behavioural Processes. 136: 64–72. doi:10.1016/j.beproc.2017.01.011.
- 1 2 Van Valkenburgh, Blaire; Hertel, Fritz (1993). "Tough Times at La Brea: Tooth Breakage in Large Carnivores of the Late Pleistocene" (PDF). Science, New Series. American Association for the Advancement of Science. 261 (5120): 456–459. Bibcode:1993Sci...261..456V. doi:10.1126/science.261.5120.456. PMID 17770024.
- ↑ Therrien, François (2005). "Mandibular force profiles of extant carnivorans and implications for the feeding behaviour of extinct predators". Journal of Zoology. 267 (3): 249. doi:10.1017/S0952836905007430.
- 1 2 3 Van Valkenburgh, B (1988). "Incidence of tooth breakage among large predatory mammals". Am. Nat. 131 (2): 291–302. doi:10.1086/284790.
- ↑ DeSantis, L.R.G.; Schubert, B.W.; Schmitt-Linville, E.; Ungar, P.; Donohue, S.; Haupt, R.J. (September 15, 2015). John M. Harris, ed. "Dental microwear textures of carnivorans from the La Brea Tar Pits, California and potential extinction implications" (PDF). Science Series 42. Contributions in Science (A special volume entitled La Brea and Beyond: the Paleontology of Asphalt-Preserved Biotas in commemoration of the 100th anniversary of the Natural History Museum of Los Angeles County's excavations at Rancho La Brea). Natural History Museum of Los Angeles County: 37–52.
- ↑ Leonard, Jennifer A.; Vilà, Carles; Fox-Dobbs, Kena; Koch, Paul L.; Wayne, Robert K.; Van Valkenburgh, Blaire (2007). "Megafaunal Extinctions and the Disappearance of a Specialized Wolf Ecomorph" (PDF). Current Biology. 17 (13): 1146. doi:10.1016/j.cub.2007.05.072. PMID 17583509.
- ↑ Aggarwal, R. K.; Kivisild, T.; Ramadevi, J.; Singh, L. (2007). "Mitochondrial DNA coding region sequences support the phylogenetic distinction of two Indian wolf species" (PDF). Journal of Zoological Systematics and Evolutionary Research. 45 (2): 163–172. doi:10.1111/j.1439-0469.2006.00400.x. Archived from the original (PDF) on 2012-03-04.
- ↑ Jhala, Y.; Sharma, D. K. (2004). "The Ancient Wolves of India" (PDF). International Wolf. 14 (2): 15–16. Archived from the original (PDF) on 2009-04-21.
- ↑ Chambers SM, Fain SR, Fazio B, Amaral M (2012). "An account of the taxonomy of North American wolves from morphological and genetic analyses". North American Fauna. 77: 1–67. doi:10.3996/nafa.77.0001.
- ↑ Wilson, D.E.; Reeder, D.M., eds. (2005). "Genus Canis". Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. ISBN 978-0-8018-8221-0. OCLC 62265494.
- ↑ Lindblad-Toh, K.; Wade, C. M.; Mikkelsen, T. S.; Karlsson, E. K.; Jaffe, D. B.; Kamal, M.; Clamp, M.; Chang, J. L.; Kulbokas, E. J.; Zody, M. C.; Mauceli, E.; Xie, X.; Breen, M.; Wayne, R. K.; Ostrander, E. A.; Ponting, C. P.; Galibert, F.; Smith, D. R.; Dejong, P. J.; Kirkness, E.; Alvarez, P.; Biagi, T.; Brockman, W.; Butler, J.; Chin, C. W.; Cook, A.; Cuff, J.; Daly, M. J.; Decaprio, D.; et al. (2005). "Genome sequence, comparative analysis and haplotype structure of the domestic dog". Nature. 438 (7069): 803–819. Bibcode:2005Natur.438..803L. doi:10.1038/nature04338. PMID 16341006.
- 1 2 3 Koepfli, K.-P.; Pollinger, J.; Godinho, R.; Robinson, J.; Lea, A.; Hendricks, S.; Schweizer, R. M.; Thalmann, O.; Silva, P.; Fan, Z.; Yurchenko, A. A.; Dobrynin, P.; Makunin, A.; Cahill, J. A.; Shapiro, B.; Álvares, F.; Brito, J. C.; Geffen, E.; Leonard, J. A.; Helgen, K. M.; Johnson, W. E.; O'Brien, S. J.; Van Valkenburgh, B.; Wayne, R. K. (2015-08-17). "Genome-wide Evidence Reveals that African and Eurasian Golden Jackals Are Distinct Species". Current Biology. 25 (16): 2158–65. doi:10.1016/j.cub.2015.06.060. PMID 26234211.
- ↑ Sharma, D. K.; Maldonado, J. E.; Jhala, Y. V.; Fleischer, R. C. (2004). "Ancient wolf lineages in India". Proceedings of the Royal Society B: Biological Sciences. 271 (Suppl 3): S1–S4. doi:10.1098/rsbl.2003.0071. PMC 1809981. PMID 15101402.
- ↑ Aggarwal, R. K.; Kivisild, T.; Ramadevi, J.; Singh, L. (2007). "Mitochondrial DNA coding region sequences support the phylogenetic distinction of two Indian wolf species". Journal of Zoological Systematics and Evolutionary Research. 45 (2): 163–172. doi:10.1111/j.1439-0469.2006.00400.x.
- ↑ Werhahn, Geraldine; Senn, Helen; Kaden, Jennifer; Joshi, Jyoti; Bhattarai, Susmita; Kusi, Naresh; Sillero-Zubiri, Claudio; MacDonald, David W. (2017). "Phylogenetic evidence for the ancient Himalayan wolf: Towards a clarification of its taxonomic status based on genetic sampling from western Nepal". Royal Society Open Science. 4 (6): 170186. Bibcode:2017RSOS....470186W. doi:10.1098/rsos.170186.
- ↑ vonHoldt, Bridgett M.; Driscoll, Carlos A. (2016). "3-Origins of the dog:Genetic insights into dog domestication". In James Serpell. The Domestic Dog: Its Evolution, Behavior and Interactions with People (2nd ed.). Cambridge University Press. pp. 22–41. ISBN 978-1-107-02414-4.
- ↑ Werdelin, Lars; Lewis, Margaret E (2005). "Plio-Pleistocene Carnivora of eastern Africa: Species richness and turnover patterns". Zoological Journal of the Linnean Society. 144 (2): 121. doi:10.1111/j.1096-3642.2005.00165.x.
- ↑ Koepfli, Klaus-Peter; Pollinger, John; Godinho, Raquel; Robinson, Jacqueline; Lea, Amanda; Hendricks, Sarah; Schweizer, Rena M.; Thalmann, Olaf; Silva, Pedro; Fan, Zhenxin; Yurchenko, Andrey A.; Dobrynin, Pavel; Makunin, Alexey; Cahill, James A.; Shapiro, Beth; Álvares, Francisco; Brito, José C.; Geffen, Eli; Leonard, Jennifer A.; Helgen, Kristofer M.; Johnson, Warren E.; o'Brien, Stephen J.; Van Valkenburgh, Blaire; Wayne, Robert K. (2015). "Genome-wide Evidence Reveals that African and Eurasian Golden Jackals Are Distinct Species". Current Biology. 25 (16): 2158. doi:10.1016/j.cub.2015.06.060. PMID 26234211.
- ↑ Amri, Lamjed; Bartolini Lucenti, Saverio; Mtimet, Moncef Saïd; Karoui-Yaakoub, Narjess; Ros-Montoya, Sergio; Espigares, Maria-Patrocinio; Boughdiri, Mabrouk; Bel Haj Ali, Nebiha; Martínez-Navarro, Bienvenido (2017). "Canis othmanii sp. nov. (Carnivora, Canidae) from the early Middle Pleistocene site of Wadi Sarrat (Tunisia)". Comptes Rendus Palevol. 16 (7): 774. doi:10.1016/j.crpv.2017.05.004.
| Wikispecies has information related to Canis |