Acetylcholinesterase inhibitor
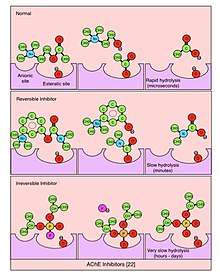
An acetylcholinesterase inhibitor (often abbreviated AChEI) or anti-cholinesterase is a chemical or a drug that inhibits the acetylcholinesterase enzyme from breaking down acetylcholine, thereby increasing both the level and duration of action of the neurotransmitter acetylcholine. Acetylcholinesterase inhibitors are classified as reversible, irreversible, or quasi-irreversible (also called pseudo-irreversible).
Uses
Acetylcholinesterase inhibitors:[1]
- Occur naturally as venoms and poisons
- Are used as weapons in the form of nerve agents
- Are used as insecticides
- Are used medicinally:
- To treat myasthenia gravis. In myasthenia gravis, they are used to increase neuromuscular transmission.
- To treat glaucoma
- To treat postural tachycardia syndrome
- As an antidote to anticholinergic poisoning
- To reverse the effect of non-depolarising muscle relaxants
- To treat neuropsychiatric symptoms of diseases such as Alzheimer's disease, particularly apathy
- To increase chances of lucid dreaming (by prolonging REM sleep)[2]
- To treat Alzheimer's disease, the Lewy body dementias and Parkinson's disease. In these neurodegenerative conditions AChEIs are primarily used to treat the cognitive (memory and learning deficits mostly) symptoms of dementia. These symptoms are attenuated due to the role of acetylcholine in cognition in the CNS. There is some evidence to suggest that AChEIs may attenuate psychotic symptoms (especially visual hallucinations) in Parkinson's disease.[3]
- To treat cognitive impairments in patients with schizophrenia. There is some evidence to suggest efficacy in treating positive, negative and affective symptoms.[4][5][6]
- As a treatment for autism and to increase the percentage of Rapid eye movement sleep in autistic children, in line with the mechanism by which they encourage lucid dreaming.[7][8]
Side effects
| Potential side effects of acetylcholinesterase inhibitors[9][10] | |||
|---|---|---|---|
| mild – usually goes away | potentially serious | ||
| |||
Some major effects of cholinesterase inhibitors:
- Actions on the parasympathetic nervous system, (the parasympathetic branch of the autonomic nervous system) may cause bradycardia, hypotension, hypersecretion, bronchoconstriction, GI tract hypermotility, and decrease intraocular pressure, increase lower esophageal sphincter (LES) tone
- SLUDGE syndrome.
- Actions on the neuromuscular junction will result in prolonged muscle contraction.
- The effects of neostigmine on postoperative nausea and vomiting are controversial and there is not a clear linkage in clinical practice, however, there is good evidence to support the reduction in risk when anticholinergic agents are administered.[11]
Administration of reversible cholinoesterase inhibitors is contraindicated with those that have urinary retention due to obstruction.
Titration phase
When used in the central nervous system to alleviate neurological symptoms, such as rivastigmine in Alzheimer's disease, all cholinesterase inhibitors require doses to be increased gradually over several weeks, and this is usually referred to as the titration phase. Many other types drug treatments may require a titration or stepping up phase. This strategy is used to build tolerance to adverse events or to reach a desired clinical effect.[12] This also prevents accidental overdose and is therefore recommended when initiating treatment with drugs that are extremely potent and/or toxic (drugs with a low therapeutic index).
Examples
Reversible inhibitor
Compounds which function as reversible competitive or noncompetitive inhibitors of cholinesterase are those most likely to have therapeutic uses. These include:
- Some organophosphates not listed under "Irreversible" below
- Carbamates
- Phenanthrene derivatives
- Caffeine – noncompetitive (also an Adenosine receptor antagonist)[13]
- Rosmarinic acid - ester of Caffeic acid. Found in plants species of family Lamiaceae.[14]
- Alpha-Pinene - noncompetitive reversible [15][16]
- Piperidines
- Tacrine, also known as tetrahydroaminoacridine (THA')
- Edrophonium
- Huperzine A[17][18]
- Ladostigil
- Ungeremine[19]
- Lactucopicrin
- Acotiamide
- Hybrid/bitopic ligands[20]
Comparison table
| Inhibitor | Duration | Main site of action | Clinical use | Adverse effects |
|---|---|---|---|---|
| Edrophonium | short (10 min.)[21] | neuromuscular junction[21] | diagnosis of myasthenia gravis[21] | |
| Neostigmine | medium (1–2 hrs.)[21] | neuromuscular junction[21] |
|
visceral[21] |
| Physostigmine | medium (0.5-5 hrs.)[21] | postganglionic parasympathetic[21] | treat glaucoma (eye drops)[21] | |
| Pyridostigmine | medium (2–3 hrs.)[21] | neuromuscular junction[21] |
|
|
| Dyflos | long[21] | postganglionic parasympathetic[21] | historically to treat glaucoma (eye drops)[21] | toxic[21] |
| Echothiophate (irreversible) | long[21] | postganglionic parasympathetic[21] | treat glaucoma (eye drops)[21] | systemic effects[21] |
| Parathion (irreversible) | long[21] | none[21] | toxic[21] |
Quasi-irreversible inhibitor
Compounds which function as quasi-irreversible inhibitors of cholinesterase are those most likely to have use as chemical weapons or pesticides. These include:
- Organophosphates
- Echothiophate
- Diisopropyl fluorophosphate
- Cadusafos
- Chlorpyrifos
- Cyclosarin
- Dichlorvos
- Dimethoate
- Metrifonate (irreversible)
- Sarin
- Soman
- Tabun
- VX
- VE
- VG
- VM
- Diazinon
- Malathion
- Parathion
- Carbamates
- Aldicarb
- Bendiocarb
- Bufencarb
- Carbaryl
- Carbendazim
- Carbetamide
- Carbofuran
- Carbosulfan
- Chlorbufam
- Chloropropham
- Ethiofencarb
- Formetanate
- Methiocarb
- Methomyl
- Oxamyl
- Phenmedipham
- Pinmicarb
- Pirimicarb
- Propamocarb
- Propham
- Propoxur
- Onchidal
- Coumarins
See also
References
- ↑ Colovic, MB; Krstic, Danijela Z.; Lazarevic-Pasti, Tamara D.; Bondzic, Aleksandra M.; Vasic, Vesna M. (2013). "Acetylcholinesterase Inhibitors: Pharmacology and Toxicology". Current Neuropharmacology. 11 (3): 315–335. doi:10.2174/1570159X11311030006. PMC 3648782. PMID 24179466.
- ↑ Yuschak, Thomas (2006). Advanced Lucid Dreaming: The Power of Supplements. Lulu. ISBN 1430305428.
- ↑ Taylor, D; Paton, C; Shitij, K (2012). Maudsley Prescribing Guidelines in Psychiatry (11th ed.). West Sussex: Wiley-Blackwell. ISBN 978-0-47-097948-8.
- ↑ Singh, J; Kour, K; Jayaram, MB (January 2012). "Acetylcholinesterase inhibitors for schizophrenia". The Cochrane Database of Systematic Reviews. 1: CD007967. doi:10.1002/14651858.CD007967.pub2. PMID 22258978. Archived from the original on 2017-09-03. Lay summary.
- ↑ Choi, KH; Wykes, T; Kurtz, MM (September 2013). "Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: meta-analytical investigation of efficacy". The British Journal of Psychiatry. 203 (3): 172–178. doi:10.1192/bjp.bp.111.107359. PMC 3759029. PMID 23999481.
- ↑ Ribeiz, SR; Bassitt, DP; Arrais, JA; Avila, R; Steffens, DC; Bottino, CM (April 2010). "Cholinesterase Inhibitors as Adjunctive Therapy in Patients with Schizophrenia and Schizoaffective Disorder A Review and Meta-Analysis of the Literature". CNS Drugs. 24 (4): 303–317. doi:10.2165/11530260-000000000-00000. PMID 20297855.
- ↑ Buckley, A. W.; Sassower, K.; Rodriguez, A. J.; Jennison, K.; Wingert, K.; Buckley, J.; Thurm, A.; Sato, S.; Swedo, S. (2011). "An Open Label Trial of Donepezil for Enhancement of Rapid Eye Movement Sleep in Young Children with Autism Spectrum Disorders". Journal of Child and Adolescent Psychopharmacology. 21 (4): 353–357. doi:10.1089/cap.2010.0121. PMC 3157749. PMID 21851192.
- ↑ Handen, B. L.; Johnson, C. R.; McAuliffe-Bellin, S.; Murray, P. J.; Hardan, A. Y. (2011). "Safety and Efficacy of Donepezil in Children and Adolescents with Autism: Neuropsychological Measures". Journal of Child and Adolescent Psychopharmacology. 21 (1): 43–50. doi:10.1089/cap.2010.0024. PMC 3037196. PMID 21309696.
- ↑ Consumer Reports; Drug Effectiveness Review Project (May 2012). "Evaluating Prescription Drugs Used to Treat: Alzheimer's Disease Comparing Effectiveness, Safety, and Price" (PDF). Best Buy Drugs. Consumer Reports: 2. Archived (PDF) from the original on 5 September 2012. Retrieved 1 May 2013. , which claims Alzheimer's Association guidance as a source
- ↑ Inglis, F. (2002). "The tolerability and safety of cholinesterase inhibitors in the treatment of dementia". International journal of clinical practice. Supplement (127): 45–63. PMID 12139367.
- ↑ Barash. Clinical Anesthesia, 7th edition. Pages 552-554.
- ↑ Inglis, F (2002). "The tolerability and safety of cholinesterase inhibitors in the treatment of dementia". International journal of clinical practice. Supplement (127): 45–63. PMID 12139367.
- ↑ Karadsheh, N; Kussie, P; Linthicum, DS (1991). "Inhibition of acetylcholinesterase by caffeine, anabasine, methyl pyrrolidine and their derivatives". Toxicology letters. 55 (3): 335–42. doi:10.1016/0378-4274(91)90015-X. PMID 2003276.
- ↑ Vladimir-Knežević, Sanda; Blažeković, Biljana; Kindl, Marija; Vladić, Jelena; Lower-Nedza, Agnieszka D.; Brantner, Adelheid H. (2014-01-09). "Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family". Molecules. 19 (1): 767–782. doi:10.3390/molecules19010767. Archived from the original on 2015-12-11.
- ↑ Miyazawa, Mitsuo; Yamafuji, Chikako (2005-03-09). "Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids". Journal of Agricultural and Food Chemistry. 53 (5): 1765–1768. doi:10.1021/jf040019b. ISSN 0021-8561. PMID 15740071.
- ↑ Perry, Nicolette S. L.; Houghton, Peter J.; Theobald, Anthony; Jenner, Peter; Perry, Elaine K. (2000-07-01). "In-vitro Inhibition of Human Erythrocyte Acetylcholinesterase by Salvia lavandulaefolia Essential Oil and Constituent Terpenes". Journal of Pharmacy and Pharmacology. 52 (7): 895–902. doi:10.1211/0022357001774598. ISSN 2042-7158. PMID 10933142. Archived from the original on 2015-12-25.
- ↑ Bauer, Brent A. Alzheimer's disease Archived 2012-08-19 at the Wayback Machine.. mayoclinic.com
- ↑ Wang, BS; Wang, H; Wei, ZH; Song, YY; Zhang, L; Chen, HZ (2009). "Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis". Journal of neural transmission (Vienna, Austria : 1996). 116 (4): 457–65. doi:10.1007/s00702-009-0189-x. PMID 19221692.
- ↑ Rhee IK, I; Appels N; Hofte B; Karabatak B; Erkelens C; Stark LM; Flippin LA; Verpoorte R (November 2004). "Isolation of the Acetylcholinesterase Inhibitor Ungeremine from Nerine bowdenii by Preparative HPLC Coupled On-Line to a Flow Assay System". Biological & Pharmaceutical Bulletin. 27 (11): 1804–1809. doi:10.1248/bpb.27.1804. PMID 15516727.
- ↑ Messerer, R., Dallanoce, C., Matera, C., Wehle, S., Flammini, L., Chirinda, B., . . . Holzgrabe, U. (2017). Novel bipharmacophoric inhibitors of the cholinesterases with affinity to the muscarinic receptors M1 and M2. MedChemComm, 8(6), 1346-1359. doi:10.1039/c7md00149e
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 156
External links
- Acetylcholinesterase+inhibitors at the US National Library of Medicine Medical Subject Headings (MeSH)
- Acetylcholinesterase: A gorge-ous enzyme QUite Interesting PDB Structure article at PDBe