Uncompetitive inhibitor
Uncompetitive inhibition, also known as anti-competitive inhibition, takes place when an enzyme inhibitor binds only to the complex formed between the enzyme and the substrate (the E-S complex). Uncompetitive inhibition typically occurs in reactions with two or more substrates or products.[1]
While uncompetitive inhibition requires that an enzyme-substrate complex must be formed, non-competitive inhibition can occur with or without the substrate present.
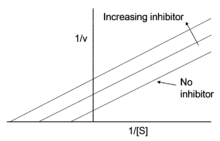
Uncompetitive inhibition is distinguished from competitive inhibition by two observations: first uncompetitive inhibition cannot be reversed by increasing [S] and second, as shown, the Lineweaver–Burk plot yields parallel rather than intersecting lines. This behavior is found in the inhibition of acetylcholinesterase by tertiary amines (R3N). Such compounds bind to the enzyme in its various forms, but the acyl-intermediate-amine complex cannot break down into enzyme plus product.[2]
Mechanism
Uncompetitive inhibition is unique in that the inhibitor binds to the enzyme-substrate complex. This could imply that the binding site for the inhibitor is accessible only after the enzyme has bound to its substrate.[3] This reduction in the effective concentration of the E-S complex increases the enzyme's apparent affinity for the substrate through Le Chatelier's principle (Km is lowered) and decreases the maximum enzyme activity (Vmax), as it takes longer for the substrate or product to leave the active site. Uncompetitive inhibition works best when substrate concentration is high. An uncompetitive inhibitor need not resemble the substrate of the reaction it is inhibiting.
Mathematical definition
The Lineweaver–Burk equation states that:
Where v is the initial reaction velocity, Km is the Michaelis–Menten constant, Vmax is the maximum reaction velocity, and [S] is the concentration of the substrate.[4]
The Lineweaver–Burk plot for an uncompetitive inhibitor produces a line parallel to the original enzyme-substrate plot, but with a higher y-intercept, due to the presence of an inhibition term :
Where [I] is the concentration of the inhibitor and Ki is an inhibition constant characteristic of the inhibitor.[5][6]
The Michaelis-Menten equation is altered to:
where
- and
As described by the equation above, at high concentrations of substrate, V0 approaches Vmax/α'. Thus, an uncompetitive inhibitor lowers the measured Vmax. Apparent Km also decreases, because [S] required to reach one-half Vmax decreases by the factor α'.[7] It is important to note that Vmax and Km decrease at the same rate as a result of the inhibitor.[8] This is apparent when viewing a Lineweaver-Burk plot of uncompetitive enzyme inhibition: the ratio between V and Km remains the same with or without an inhibitor present.
Notes and references
- ↑ Vladimir., Leskovac, (2004). Comprehensive Enzyme Kinetics. Kluwer Academic Publishers. ISBN 0306467127. OCLC 517776240.
- ↑ Mathews, Christopher K.; van Holde, Kensal E.; Appling, Dean R.; Anthony-Cahill, SpencerJ. (February 26, 2012). Biochemistry (4 ed.). Pearson. ISBN 978-0138004644.
- ↑ Athel., Cornish-Bowden, (2014). Principles of Enzyme Kinetics. Elsevier Science. ISBN 1483164675. OCLC 897021733.
- ↑ Cleland, W. W. (1963). "The kinetics of enzyme-catalyzed reactions with two or more substrates or products". Biochimica et Biophysica Acta (BBA) - Specialized Section on Enzymological Subjects. 67: 173–187. doi:10.1016/0926-6569(63)90226-8. PMID 14021668.
- ↑ Rhodes, David. "Enzyme Kinetics - Single Substrate, Uncompetitive Inhibition, Lineweaver-Burk Plot". Purdue University. Retrieved 31 August 2013.
- ↑ Cornish-Bowden, A. (1974). "A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors". The Biochemical Journal. 137 (1): 143–144. doi:10.1042/bj1370143. PMC 1166095. PMID 4206907.
- ↑ Nelson, David L.; Cox, Michael M. (November 21, 2012). Lehninger Principles of Biochemistry (6 ed.). W.H. Freeman. ISBN 978-1429234146.
- ↑ Athel., Cornish-Bowden, (2014). Principles of Enzyme Kinetics. Elsevier Science. ISBN 1483164675. OCLC 897021733.