Triethylaluminium
Triethylaluminium is an organoaluminium compound. It is actually a dimer, so despite its name, the formula for this compound is Al2(C2H5)6, shortened to Al2Et6 (Et, ethyl). This volatile, colorless liquid is highly pyrophoric, igniting immediately upon exposure to air. It is normally stored in stainless steel containers either as a pure liquid or as a solution in hydrocarbon solvents such as hexane, heptane, or toluene. Triethylaluminium is mainly used as a co-catalyst in the industrial production of polyethylene, polypropylene and for the production of medium chain alcohols.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Triethylalumane | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | TEA,[1] TEAl,[2] TEAL[3] |
| ChemSpider | |
| ECHA InfoCard | 100.002.382 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C12H30Al2 | |
| Molar mass | 228.335 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8324 g/mL at 25 °C |
| Melting point | −46 °C (−51 °F; 227 K) |
| Boiling point | 128 to 130 °C (262 to 266 °F; 401 to 403 K) at 50 mmHg |
| Hazards | |
| Main hazards | pyrophoric |
| R-phrases (outdated) | R14 R17 R34 |
| S-phrases (outdated) | S16 S43 S45 |
| NFPA 704 (fire diamond) | |
| Flash point | −18 °C (0 °F; 255 K) |
| Related compounds | |
Related compounds |
Trimethylaluminum |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and bonding

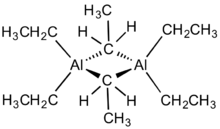
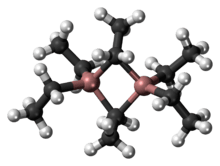
The compound is a dimer of triethylaluminium. One pair of ethyl groups bridge the two Al centers, and the other four groups are terminal ligands. The two bridging carbon centres are five-coordinate. The bonding is reminiscent of that of diborane, involving 3-centred, 2-electron bonds. As in trimethylaluminium, triethylaluminium is structurally fluctional resulting in rapid interchange of the terminal and bridging ethyl groups. At higher temperatures, the dimer cracks into monomeric AlEt3.[4]
Synthesis and reactions
Triethylaluminium can be formed via several routes. The discovery of an efficient route was a significant technological achievement. The multistep process uses aluminium metal, hydrogen gas, and ethylene, summarized as follows:[5]
- 2 Al + 3 H2 + 6 C2H4 → Al2Et6
Because of this efficient synthesis, triethylaluminium is one of the most available organoaluminium compounds.
Triethylaluminium can also be generated from ethylaluminium sesquichloride (Al2Cl3Et3), which arises by treating aluminium powder with chloroethane. Reduction of ethylaluminium sesquichloride with an alkali metal such as sodium gives triethylaluminium:[6]
- 3 Al2Cl3Et3 + 9 Na → 2 Al2Et6 + 2 Al + 9 NaCl
Reactivity
The Al–C bonds of triethylaluminium are polarized to such an extent that the carbon is easily protonated, releasing ethane:[7]
- Al2Et6 + 6 HX → 2 AlX3 + 6 EtH
For this reaction, even weak acids can be employed such as terminal acetylenes and alcohols.
The linkage between the pair of aluminium centres is relatively weak and can be cleaved by Lewis bases (L) to give adducts with the formula AlEt3L:
- Al2Et6 + 2 L → 2 LAlEt3
Applications
Precursors to fatty alcohols
Triethylaluminium is used industrially as an intermediate in the production of fatty alcohols, which are converted to detergents. The first step involves the oligomerization of ethylene by the Aufbau reaction, which gives a mixture of trialkylaluminium compounds (simplified here as octyl groups):[5]
- Al2(C2H5)6 + 18 C2H4 → Al2(C8H17)6
Subsequently, these trialkyl compounds are oxidized to aluminium alkoxides, which are then hydrolysed:
- Al2(C8H17)6 + 3 O2 → Al2(OC8H17)6
- Al2(OC8H17)6 + 6 H2O → 6 C8H17OH + 2 "Al(OH)3"
Co-catalysts in olefin polymerization
Large amount of TEAL and related aluminium alkyls are used in Ziegler-Natta catalysis. They serve to activate the transition metal catalyst both as a reducing agent and an alkylating agent. TEAL also functions to scavenge water and oxygen.[8]
Reagent in organic and organometallic chemistry
Triethylaluminium has niche uses as a precursor to other organoaluminium compounds, such as diethylaluminium cyanide:[9]
Pyrophoric agent
Triethylaluminium ignites on contact with air and will ignite and/or decompose on contact with water, and with any other oxidizer[10]—it is one of the few substances sufficiently pyrophoric to ignite on contact with cryogenic liquid oxygen. The enthalpy of combustion, ΔcH°, is –5105.70 ± 2.90 kJ/mol[11] (–22.36 kJ/g). Its easy ignition makes it particularly desirable as a rocket engine ignitor. The SpaceX Falcon 9 rocket uses a triethylaluminium-triethylborane mixture as a first-stage ignitor.[1]
Triethylaluminium thickened with polyisobutylene is used as an incendiary weapon, as a pyrophoric alternative to napalm; e.g., in the M74 clip holding four rockets for the M202A1 launchers.[12] In this application it is known as TPA, for thickened pyrotechnic agent or thickened pyrophoric agent. The usual amount of the thickener is 6%. The amount of thickener can be decreased to 1% if other diluents are added. For example, n-hexane, can be used with increased safety by rendering the compound non-pyrophoric until the diluent evaporates, at which point a combined fireball results from both the triethylaluminium and the hexane vapors.[13] The M202 was withdrawn from service in the mid-1980s owing to safety, transport, and storage issues. Some saw limited use in the Afghanistan War against caves and fortified compounds.
See also
- Triethylborane, used as an ignitor in the Pratt & Whitney J58 turbojet/ramjet engines.
- Trimethylaluminium
References
- Mission Status Center, June 2, 2010, 1905 GMT, SpaceflightNow, accessed 2010-06-02, Quotation: "The flanges will link the rocket with ground storage tanks containing liquid oxygen, kerosene fuel, helium, gaserous nitrogen and the first stage ignitor source called triethylaluminum-triethylborane, better known as TEA-TAB."
-
"Gulbrandsen Chemicals, Metal Alkyls: Triethylaluminum (TEAl)". Gulbrandsen. Retrieved December 12, 2017.
Triethylaluminum (TEAl) is a pyrophoric liquid...
- Malpass, Dennis B.; Band, Elliot (2012). Introduction to Industrial Polypropylene: Properties, Catalysts Processes. John Wiley & Sons. ISBN 9781118463208.
- Gábor Vass, György Tarczay, Gábor Magyarfalvi, András Bödi, and László Szepes “HeI Photoelectron Spectroscopy of Trialkylaluminum and Dialkylaluminum Hydride Compounds and Their Oligomers” Organometallics, 2002, volume 21, pp. 2751–2757. doi:10.1021/om010994h
- Michael J. Krause, Frank Orlandi, Alfred T. Saurage, Joseph R. Zietz Jr. “Aluminum Compounds, Organic” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_543
- Krause, M. J; Orlandi, F; Saurage, A T.; Zietz, J R, "Organic Aluminum Compounds" Wiley-Science 2002.
- Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2
- Dennis B. Malpass (2010). "Commercially Available Metal Alkyls and Their Use in Polyolefin Catalysts". In Ray Hoff, Robert T. Mathers (eds.). Handbook of Transition Metal Polymerization Catalysts. John Wiley & Sons, Inc. doi:10.1002/9780470504437.ch1.CS1 maint: uses editors parameter (link)
- Wataru Nagata and Yoshioka Mitsuru (1988). "Diethylaluminum Cyanides". Organic Syntheses.; Collective Volume, 6, p. 436
- TEA Material Safety Data Sheet Archived 2006-11-14 at the Wayback Machine, accessed March 27, 2007
- https://www.chemeo.com/cid/63-022-7/Triethylaluminum
- M202A1 Flame Assault Shoulder Weapon (Flash), inetres.com
- Encyclopedia of Explosives and Related Items, Vol.8, US Army
