Thiocarbonic acid
Thiocarbonic acid is an inorganic acid which is related to carbonic acid. It is an unstable red oil with the chemical formula H2CS3. It is often referred to as trithiocarbonic acid so as to differentiate it from other thiocarbonates.
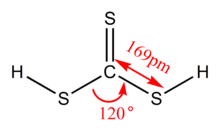 | |
| Names | |
|---|---|
| IUPAC name
carbonotrithioic acid | |
| Other names
Trithiocarbonic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.931 |
| EC Number |
|
| MeSH | C013321 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| CH2S3 | |
| Molar mass | 110.22 g/mol |
| Appearance | red, oily liquid |
| Density | 1.483 g/cm3(liquid) |
| Melting point | −26.8 °C; −16.3 °F; 246.3 K |
| Boiling point | 58 °C; 136 °F; 331 K |
| Related compounds | |
Related compounds |
Carbonic acid, Thiosulfuric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Discovery and synthesis
It was first reported in brief by Zeise in 1824 and later in more detail by Berzelius in 1826,[1] in both cases it was produced by the action of carbon disulfide on a hydrosulfide salt (e.g. potassium hydrosulfide).[2]
- CS2 + 2 KSH → K2CS3 + H2S
Treatment with acids liberates the thiocarbonic acid as a red oil
- K2CS3 + 2 HX → H2CS3 + 2 KX
Both the acid and many of its salts are unstable and decompose via the release of carbon disulfide, particularly upon heating:
- H2CS3 → CS2 + H2S
Applications
Thiocarbonic acid currently has no significant applications. It's esters, which are sometimes called thioxanthates, find use in RAFT polymerization.
References
- Berzelius, J. J. (1826). "Ueber die Schwefelsalze" [About the sulfur salts]. Annalen der Physik (in German). 82 (4): 425–458. doi:10.1002/andp.18260820404.
- O'Donoghue, Ida Guinevere; Kahan, Zelda (1906). "CLXXIV.—Thiocarbonic acid and some of its salts". J. Chem. Soc., Trans. 89: 1812–1818. doi:10.1039/CT9068901812.