Sarecycline
Sarecycline (trade name Seysara; development code WC-3035) is a narrow-spectrum tetracycline-derived antibiotic.[1] In the United States, it was approved by the FDA in October 2018 for the treatment of moderate to severe acne vulgaris.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Seysara |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.241.852 |
| Chemical and physical data | |
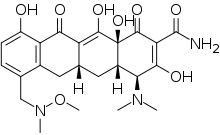
| Formula | C24H29N3O8 |
| Molar mass | 487.509 g·mol−1 |
| 3D model (JSmol) | |
| |
Paratek Pharmaceuticals, Inc. licensed the US rights to sarecycline for the treatment of acne in the United States to Actavis, a subsidiary of Allergan, while retaining rights in the rest of the world.[3]
Allergan initiated a Phase 3 study in December 2014 evaluating the efficacy and safety of sarecycline tablets 1.5 mg/kg per day taken orally for 12 weeks versus placebo in the treatment of acne vulgaris.[4] Two phase 3 randomized, multi-center, double-blind, placebo-controlled studies evaluating the efficacy and safety of sarecycline in moderate to severe acne reported positive results on 27 March 2017.[5]
References
- Zhanel G, Critchley I, Lin LY, Alvandi N (January 2019). "Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris". Antimicrobial Agents and Chemotherapy. 63 (1). doi:10.1128/AAC.01297-18. PMC 6325184. PMID 30397052.
- "FDA Approves Sarecycline for Moderate to Severe Acne". MedScape. October 2, 2018.
- "Paratek Pharmaceuticals Inc". Bloomberg.
- Clinical trial number NCT02320149 for "Study to Evaluate Safety & Efficacy of Sarecycline in Treatment of Acne" at ClinicalTrials.gov
- "Allergan and Paratek Announce Positive Results From Two Phase 3 Trials of Sarecycline for the Treatment of Moderate to Severe Acne". www.globenewswire.com. 27 March 2017. Retrieved 16 May 2017.