Eugeroic
Eugeroics (originally, "eugrégorique" or "eugregoric"),[1] also known as wakefulness-promoting agents and wakefulness-promoting drugs, are a class of drugs that promote wakefulness and alertness.[2][3] They are medically indicated for the treatment of certain sleep disorders including excessive daytime sleepiness (EDS) in narcolepsy or obstructive sleep apnea (OSA).[2][3] They generally have a very low addictive potential.[2][3] Eugeroics are also often prescribed off-label for the treatment of EDS in idiopathic hypersomnia,[4] a rare and often debilitating sleep disorder which currently has no official treatments approved by the Food and Drug Administration (FDA).
| Eugeroic | |
|---|---|
| Drug class | |
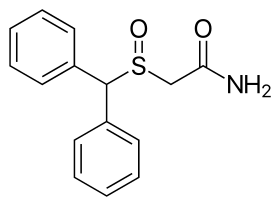 The chemical structure of modafinil, the prototypical drug of this class. | |
| Class identifiers | |
| Synonyms | wakefulness-promoting agent wakefulness-promoting drug |
| Use | Promote wakefulness and alertness |
| ATC code | N06B |
| In Wikidata | |
Modafinil and armodafinil each act as a selective, weak, atypical dopamine reuptake inhibitor (DRI)[2][3] whereas adrafinil acts as a prodrug for modafinil. Other eugeroics include solriamfetol, which acts as a norepinephrine–dopamine reuptake inhibitor (NDRI), and pitolisant, which acts as a histamine 3 (H₃) receptor antagonist/inverse agonist.
Examples
Marketed
- Armodafinil (Nuvigil)
- Modafinil (Provigil)
- Solriamfetol (Sunosi)[5]
- Pitolisant (Wakix)[6]
Discontinued
Never marketed
- Flmodafinil (CRL-40,940)
- Fluorafinil (CRL-40,941)
- Fluorenol
In development
- Selective H₃ receptor antagonists/inverse agonists
- Selective orexin receptor agonists (two are currently under development)[7]
See also
- Stimulants
- Sodium oxybate
- Protriptyline
- Venlafaxine
- Selegiline
- Circadian rhythm
- International Classification of Sleep Disorders (ICSD)
Categories
References
- Milgram, Norton W.; Callahan, Heather; Siwak, Christina (2006). "Adrafinil: A Novel Vigilance Promoting Agent". CNS Drug Reviews. 5 (3): 193–212. doi:10.1111/j.1527-3458.1999.tb00100.x. ISSN 1080-563X.
- "Provigil: Prescribing information" (PDF). United States Food and Drug Administration. Cephalon, Inc. January 2015. Retrieved 16 August 2015.
- "Nuvigil: Prescribing information" (PDF). United States Food and Drug Administration. Cephalon, Inc. April 2015. Retrieved 16 August 2015.
- "Practice Parameters for the Treatment of Narcolepsy and other Hypersomnias of Central Origin" (PDF). American Academy of Sleep Medicine (AASM). September 2007.
- "What is SUNOSI® (solriamfetol) Treatment ? | SUNOSI® for Patients". www.sunosi.com. Retrieved 2020-01-03.
- "How WAKIX Works | WAKIX® (pitolisant) tablets". wakix.com. Retrieved 2020-01-03.
- "New Data Presented at World Sleep Congress Demonstrate Early Signs of Efficacy for TAK-925, a Selective Orexin Type-2 Receptor (OX2R) Agonist, in Patients with Narcolepsy Type 1". www.takeda.com. Retrieved 2019-12-06.