Dipotassium phosphate
Dipotassium phosphate (K2HPO4) (also dipotassium hydrogen orthophosphate; potassium phosphate dibasic) is the inorganic compound with the formula K2HPO4.(H2O)x (x = 0, 3, 6). Together with monopotassium phosphate (KH2PO4.(H2O)x) it is often used as a fertilizer, food additive, and buffering agent.[1]
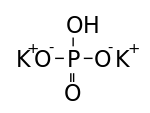 | |
| Names | |
|---|---|
| IUPAC names
Potassium hydrogenphosphate Potassium hydrogen(tetraoxidophosphate)(2−) | |
| Systematic IUPAC name
Potassium hydroxidotrioxidophosphate(2−) | |
| Other names
Potassium monohydrogen phosphate Phosphoric acid dipotassium salt Potassium phosphate dibasic | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.940 |
| EC Number |
|
| E number | E340(ii) (antioxidants, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| K2HPO4 | |
| Molar mass | 174.2 g/mol |
| Appearance | white powder deliquescent |
| Odor | odorless |
| Density | 2.44 g/cm3 |
| Melting point | > 465 °C (869 °F; 738 K) decomposes |
| 149.25 g/100 mL (20 °C) | |
| Solubility | slightly soluble in alcohol |
| Acidity (pKa) | 12.4 |
| Basicity (pKb) | 6.8 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other cations |
Disodium phosphate Diammonium phosphate |
Related compounds |
Monopotassium phosphate Tripotassium phosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
A dipotassium phosphate solution is formed by the stoichiometric reaction of phosphoric acid with two equivalents of potassium hydroxide:
- H3PO4 + 2 KOH → K2HPO4 + 2 H2O
Uses
As a food additive, dipotassium phosphate is used in imitation dairy creamers, dry powder beverages, mineral supplements, and starter cultures.[2]
As a food additive, dipotassium phosphate is categorized by the United States Food and Drug Administration as generally recognized as safe (GRAS).[3]
References
- Klaus Schrödter; Gerhard Bettermann; Thomas Staffel; Friedrich Wahl; Thomas Klein; Thomas Hofmann (2012). "Phosphoric Acid and Phosphates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_465.pub3.
- John H. Thorngate III; Seppo Salminen; Larry A. Branen; Michael P. Davidson, eds. (2001). "Food Phosphates". Food Additives. CRC Press. doi:10.1201/9780824741709.ch25. ISBN 978-0-8247-9343-2.
- "Database of Select Committee on GRAS Substances (SCOGS) Reviews". Retrieved 2008-03-22. (listed as "potassium phosphate, dibasic")
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
