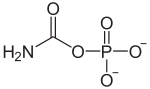
Carbamoyl phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals, it is an intermediary metabolite in nitrogen disposal through the urea cycle and the synthesis of pyrimidines. Its enzymatic counterpart, carbamoyl phosphate synthetase I (CPS I), interacts with a class of molecules called sirtuins, NAD dependent protein deacetylases, and ATP to form carbamoyl phosphate. CP then enters the urea cycle in which it reacts with ornithine (a process catalyzed by the enzyme ornithine transcarbamylase) to form citrulline. A defect in the CPS I enzyme, and a subsequent deficiency in the production of carbamoyl phosphate has been linked to hyper-ammonemia in humans.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(Carbamoyloxy)phosphonic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.230.975 |
| KEGG | |
| MeSH | Carbamoyl+phosphate |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| CH2NO5P2− | |
| Molar mass | 141.020 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
It is produced from bicarbonate, ammonia (derived from amino acids), and phosphate (from ATP). The synthesis is catalyzed by the enzyme carbamoyl phosphate synthetase, as follows:
- HCO−
3 + ATP → ADP + HO–C(O)–OPO2−
3 (carboxyl phosphate) - HO–C(O)–OPO2−
3 + NH3 + OH− → HPO2−
4 + −O–C(O)NH2 + H2O - −O–C(O)NH2 + ATP → ADP + H
2NC(O)OPO2−
3
References
- Nakagawa, Takashi; Lomb, David J.; Haigis, Marcia C.; Guarente, Leonard (2009-05-01). "SIRT5 Deacetylates Carbamoyl Phosphate Synthetase 1 and Regulates the Urea Cycle". Cell. 137 (3): 560–570. doi:10.1016/j.cell.2009.02.026. ISSN 0092-8674. PMC 2698666. PMID 19410549.
- Nelson, David L.; Cox, Michael M. (2005). Lehninger Principles of Biochemistry fourth edition. New York: W. H. Freeman and company. ISBN 978-0716743392.
- "SIRT5 Deacetylates Carbamoyl Phosphate Synthetase 1 and Regulates the Urea Cycle". Cell. 137 (3): 560–570. 2009-05-01. doi:10.1016/j.cell.2009.02.026. ISSN 0092-8674.