Citrulline
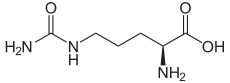
The organic compound citrulline is an α-amino acid. Its name is derived from "citrullus", the Latin word for watermelon, from which it was first isolated in 1914 by Koga and Odake.[2] It was finally identified by Wada in 1930.[3] It has the formula H2NC(O)NH(CH2)3CH(NH2)CO2H. It is a key intermediate in the urea cycle, the pathway by which mammals excrete ammonia by converting it into urea. Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-5-(carbamoylamino)pentanoic acid[1] | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 3DMet | |
| 1725417, 1725415 R, 1725416 S | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.145 |
| EC Number |
|
| 774677 S | |
| KEGG | |
| MeSH | Citrulline |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H13N3O3 | |
| Molar mass | 175.188 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| log P | −1.373 |
| Acidity (pKa) | 2.508 |
| Basicity (pKb) | 11.489 |
| Thermochemistry | |
Heat capacity (C) |
232.80 J K−1 mol−1 |
Std molar entropy (S |
254.4 J K−1 mol−1 |
| Related compounds | |
Related alkanoic acids |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
Citrulline is made from ornithine and carbamoyl phosphate in one of the central reactions in the urea cycle. It is also produced from arginine as a byproduct of the reaction catalyzed by NOS family (NOS; EC 1.14.13.39).[5] It is made from arginine by the enzyme trichohyalin at the inner root sheath and medulla of hair follicles.[6] Arginine is first oxidized into N-hydroxyl-arginine, which is then further oxidized to citrulline concomitant with release of nitric oxide.
Function
Several proteins contain citrulline as a result of a posttranslational modification. These citrulline residues are generated by a family of enzymes called peptidylarginine deiminases (PADs), which convert arginine into citrulline in a process called citrullination or deimination with the help of calcium ion. Proteins that normally contain citrulline residues include myelin basic protein (MBP), filaggrin, and several histone proteins, whereas other proteins, such as fibrin and vimentin are susceptible to citrullination during cell death and tissue inflammation.
Circulating citrulline concentration is a biomarker of intestinal functionality.[7][8]
See also
References
- "Citrulline - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification. Retrieved 1 May 2012.
- Fragkos, Konstantinos C.; Forbes, Alastair (September 2011). "Was citrulline first a laxative substance? The truth about modern citrulline and its isolation" (PDF). Nihon Ishigaku Zasshi. [Journal of Japanese History of Medicine]. 57 (3): 275–292. ISSN 0549-3323. PMID 22397107.
- Fearon, William Robert (1939). "The Carbamido Diacetyl Reaction: A Test For Citrulline". Biochemical Journal. 33 (6): 902–907. doi:10.1042/bj0330902. PMC 1264464. PMID 16746990.
- "Nos2 - Nitric Oxide Synthase". Uniprot.org. Uniprot Consortium. Retrieved 10 February 2015.
- Cox M, Lehninger AL, Nelson DR (2000). Lehninger principles of biochemistry (3rd ed.). New York: Worth Publishers. p. 449. ISBN 978-1-57259-153-0. Retrieved 13 March 2020.
- Rogers, G. E.; Rothnagel, J. A. (1983). "A sensitive assay for the enzyme activity in hair follicles and epidermis that catalyses the peptidyl-arginine-citrulline post-translational modification". Current Problems in Dermatology. 11: 171–184. doi:10.1159/000408673. ISBN 978-3-8055-3752-0. PMID 6653155.
- Fragkos, Konstantinos C.; Forbes, Alastair (2017-10-12). "Citrulline as a marker of intestinal function and absorption in clinical settings: A systematic review and meta-analysis". United European Gastroenterology Journal. 6 (2): 181–191. doi:10.1177/2050640617737632. PMC 5833233. PMID 29511548.
- Crenn, P.; et al. (2000). "Post-absorptive plasma citrulline concentration is a marker of intestinal failure in short bowel syndrome patients". Gastroenterology. 119 (6): 1496–505. doi:10.1053/gast.2000.20227. PMID 11113071.