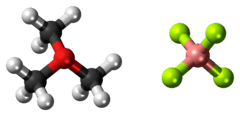
Trimethyloxonium tetrafluoroborate
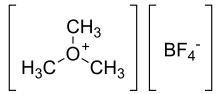 | |
 | |
| Names | |
|---|---|
| IUPAC name
Trimethyloxonium tetrafluoroborate | |
| Other names
Trimethyloxonium fluoborate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.360 |
PubChem CID |
|
| |
| |
| Properties | |
| C3H9BF4O | |
| Molar mass | 147.91 g·mol−1 |
| Melting point | 179.6–180 °C (355.3–356.0 °F; 452.8–453.1 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trimethyloxonium tetrafluoroborate is the organic compound with the formula Me3OBF4. It is sometimes called the "Meerwein salt" after Hans Meerwein.[1][2]) This salt is a strong methylating agent, being a synthetic equivalent of CH3(+). It is a white solid that rapidly degrades upon exposure to atmospheric moisture, although it is robust enough to be weighed and dispensed quickly without the benefit of inert atmosphere protection. Triethyloxonium tetrafluoroborate is a closely related reagent.
Preparation and reactions
The compound is prepared by the reaction of boron trifluoride with dimethyl ether and epichlorohydrin:[1]
- 4 Me2O·BF3 + 2 Me2O + 3 C2H3(O)CH2Cl → 3 Me3O+BF−
4 + B[(OCH(CH2Cl)CH2OMe]3
The salt hydrolyzes readily:
- Me3OBF4 + H2O → Me2O + MeOH + HBF4
Trimethyloxonium tetrafluoroborate is generally ranked as the strongest commercially available reagent for electrophilic methylation, being stronger than methyl sulfonate esters, including methyl triflate and methyl fluorosulfonate (`magic methyl').[3] Only the exotic dimethylhalonium reagents (Me2X+SbF6–, X = Cl, Br, I), methyl carboranate reagents, and the transiently-generated methyldiazonium cation (MeN+≡N) are stronger sources of electrophilic methyl.
Due to its high reactivity, it is rapidly destroyed by atmospheric moisture and best stored in an inert atmosphere glovebox at –20 °C. Its degradation products are corrosive, although it is considerably less hazardous than methyl triflate or fluorosulfonate, on account of its lack of volatility.
References
- 1 2 T. J. Curphey (1988). "Trimethyloxonium Tetrafluoroborate". Organic Syntheses. ; Collective Volume, 6, p. 1019
- ↑ Meerwein's salt classically referred to triethyloxonium tetrafluoroborate. However, in recent years, the trimethyloxonium salt has also been called Meerwein's salt.
- ↑ Stang, Peter J.; Hanack, Michael; Subramanian, L. R. (1982). "Perfluoroalkanesulfonic Esters: Methods of Preparation and Applications in Organic Chemistry". Synthesis. 1982 (02): 85–126. doi:10.1055/s-1982-29711. ISSN 0039-7881.