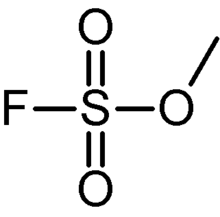
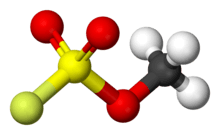
Methyl fluorosulfonate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Methyl fluorosulfonate | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.369 |
PubChem CID |
|
| |
| |
| Properties | |
| CH3FS | |
| Molar mass | 66.09 g·mol−1 |
| Density | 1.45 g/mL |
| Boiling point | 93 °C (199 °F; 366 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl fluorosulfonate, also known as magic methyl, is the organic compound with the formula FSO2OCH3. It is a colorless liquid that is used as a strong methylating agent in organic synthesis. Because of its extreme toxicity, it has largely been replaced by the related reagent methyl trifluoromethanesulfonate.
Synthesis and reactions
It is prepared by distillation of an equimolar mixture of fluorosulfonic acid and dimethyl sulfate. It was originally produced by the reaction of methanol with fluorosulfonic acid.[1]
Methyl fluorosulfonate is a highly electrophilic reagent for methylation. It is ranked as less powerful than methyl trifluoromethanesulfonate.[2]
Toxicity
References
- 1 2 Meyer, Julius; Schramm, Georg (1932). "Ester der Fluorsulfonsäure (Esters of fluorosulfonic acid)". Zeitschrift für Anorganische und Allgemeine Chemie. 206: 24–30. doi:10.1002/zaac.19322060103.
- ↑ Stang, Peter J.; Hanack, Michael; Subramanian, L. R. (1982). "Perfluoroalkanesulfonic Esters: Methods of Preparation and Applications in Organic Chemistry". Synthesis. 1982 (02): 85–126. doi:10.1055/s-1982-29711. ISSN 0039-7881.
- ↑ Hite, M.; Rinehart, W.; Braun, W.; Peck, H. (1979). "Acute toxicity of methyl fluorosulfonate (Magic Methyl)". AIHA Journal. 40 (7): 600–603. doi:10.1080/00028897708984416. PMID 484483.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.