Tricresyl phosphate
 | |
 | |
| Names | |
|---|---|
| Other names
tricresylphosphate tri-o-cresyl phosphate TOCP tritolyl phosphate tolyl phosphate tri-o-tolyl ester of phosphoric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.014.136 |
| |
| |
| Properties | |
| C21H21O4P | |
| Molar mass | 368.37 g/mol |
| Appearance | colourless liquid |
| Melting point | −40 °C (−40 °F; 233 K) |
| Boiling point | 255 °C (491 °F; 528 K) (10 mmHg) |
| Hazards | |
| Flash point | > 225 °C (437 °F; 498 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tricresyl phosphate, abbreviated TCP, is an organophosphate compound that is used as a plasticizer and diverse other applications. It is a toxic substance that causes neuropathy through ingestion, and has been the cause of several mass poisonings in history. It is a colourless, viscous liquid, although commercial samples are typically yellow. It is virtually insoluble in water.
Production
Tricresyl phosphate is manufactured by reaction of cresols with phosphorus oxychloride:
- OPCl3 + 3 HOC6H4CH3 → OP(OC6H4CH3)3 + 3 HCl
The cresol is a mixture of three isomers (ortho, meta, and para). The fact that tricresyl phosphate is derived from a mixture and itself is a mixture ensures that it remains liquid over a wide span of temperatures.
Use
Tricresyl phosphate is used as a plasticizer in nitrocellulose, acrylate lacquers, varnishes, and in polyvinyl chloride. It is a flame retardant in plastics and rubbers. It is used as a gasoline additive as a lead scavenger for tetraethyllead.[1] It is a hydraulic fluid and a heat exchange medium. It is also used by the U.S. Navy in its pure form as a lubricant in cryogenic liquid pumps. Exploiting its hydrophobic properties, it is used for the waterproofing of materials. It is a solvent for liquid–liquid extractions, a solvent for nitrocellulose and other polymers. It is used as an antiwear and extreme pressure additive in lubricants and hydraulic fluids.[2][3]
Safety
TCP is used as an additive in turbine engine oil and can potentially get into an airliner cabin via a bleed air "fume event". Aerotoxic syndrome is the name given to the alleged health ill effects (with symptoms including memory loss, depression and schizophrenia) caused by exposure to engine chemicals although UK industry-funded studies have not made a link between TCP and longterm health issues.[4] The most dangerous isomers are considered to be those containing ortho isomers, such as tri-ortho-cresyl phosphate, TOCP. The World Health Organisation stated in 1990 that "Because of considerable variation among individuals in sensitivity to TOCP, it is not possible to establish a safe level of exposure" and "TOCP are therefore considered major hazards to human health."[5] Therefore, strenuous efforts have been made to reduce the content of the ortho isomers in commercial TCP if there is a risk of human exposure.[6] Researchers at the University of Washington found that non-ortho TCP isomers present in synthetic jet engine oils do inhibit certain enzymes.[7]
TCP is combustible, although less so than typical organic compounds.
Toxicity
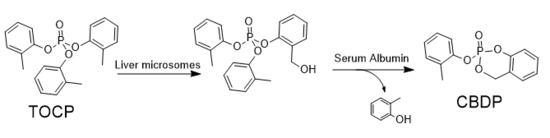
Of the three tricresyl phosphate isomers, only the ortho isomer demonstrates high toxicity. The toxicity is due to a metabolite, 2-(ortho-cresyl)-4H-1,3,2-benzodioxaphosphoran-2-one (CBDP), which is an irreversible inhibitor of human butyrylcholinesterase (BChE) and human acetylcholinesterase (AChE). CBDP ultimately binds to the BChE and AChE at a serine residue in the active site after two consecutive dealkyalation reactions (aging). Though CBDP readily inhibits BChE and AChE, BChE has a sensitivity to CBDP that is nearly one order of magnitude greater than that of AChE.[8] The inhibition of cholinesterases causes a build up in cholines that would have been broken down by the esterase. The build up of cholines causes an overactivity of neurons, ultimately causing them to function abnormally, or not at all.
Symptoms
Tri-o-cresyl phosphate poisoning is characterized by numbness of the legs and hands accompanied by weakness or paralysis of these regions, though all symptoms usually only occur after a latency period of 2–3 days. If the dose is substantial, gastrointestinal distress may occur immediately, and the latency period may be shortened. 10–40 days after the onset of numbness, there is a potential for abrupt flaccid paralysis of the toes, feet, and lower parts of the leg, which may be followed by paralysis of the fingers and hands after 4–5 additional days. Fatalities are rare, but there is potential for permanent paralysis, due to death of nerve cells and damage to myelin sheaths.[9]
Major cases
The earliest major poisoning by TOCP was in France in 1898, where 6 of 41 people treated with phosphocreosote for tuberculosis developed polyneuropathy, later to be determined to be caused by TOCP.[10]
In 1930, 50,000 cases of paralysis broke out in the midwestern and southwestern US. This was attributed to the consumption of a medicine called Jamaica Ginger, which had been adulterated with TOCP. TOCP had been mixed into the alcoholic medicine so that the drink would pass inspection by prohibition officers without making the alcohol taste as bitter as other adulterants. This caused partial paralysis of many of its consumers, characterized by an awkward, high-kneed walk, dubbed "Jake walk".[11]
In World War 2, there were several incidents in Switzerland and Germany. A military company in Magden improperly stored machine gun cooling oil – in canisters that were also used for cooking oil. 74 soldiers were poisoned and 10-12 civilians, too – because they shared rations with them. They were known as "Ölsoldaten" (oil soldiers). In the same year, a similar case occurred in central Switzerland when 17 soldiers ate salad with contaminated oil. In the Winter of 1941/1942, torpedo oil was stolen from the torpedo trial facility at Eckernförde and was, due to scarcity of proper cooking oil, used in private households. About 70 people were affected in what was known as the "Eckernförder Krankheit" (disease of Eckernförde). Similar outbreaks happened in the harbor town of Kiel (1944) and again at Eckernförde (1949). Incidentally, torpedo oil from World War 1 did not use additives, and in times of distress, it was used for cooking – without ill effects.
Another large outbreak of TOCP poisoning occurred in Meknes, Morocco, in the 1960s. Of the 10,000 cases, nearly all of them were Arab and middle class, with the majority being women. The condition was found to be caused by the adulteration of cooking oil with lubricating oil used in jet engines which contained 3% mixed cresyl phosphates.[12]
The most recent incident of large scale poisoning by TOCP was in Sri Lanka, where over 20 women suffered from acute polyneuropathy in 1977. This poisoning was determined to be caused by contamination of cooking oil that was transported in barrels that had previously stored mineral oil.[13]
References
- ↑ "Marvel & other solutions to the lead problem". Retrieved 2009-06-18.
- ↑ Svara, Jürgen; Weferling, Norbert; Hofmann, Thomas, "Phosphorus Compounds, Organic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a19_545.pub2
- ↑ Solbu, K.; Thorud, S.; Hersson, M.; Ovrebø, S.; Ellingsen, G.; Lundanes, E.; Molander, P. (Aug 2007). "Determination of airborne trialkyl and triaryl organophosphates originating from hydraulic fluids by gas chromatography-mass spectrometry. Development of methodology for combined aerosol and vapor sampling". Journal of Chromatography A. 1161 (1–2): 275–283. doi:10.1016/j.chroma.2007.05.087. ISSN 0021-9673. PMID 17574560.
- ↑ "UK Parliament: Elements of healthy cabin air".
- ↑ "Tricresyl Phosphate Environmental Health Criteria A110". International Programme On Chemical Safety. WHO Geneva. 1990.
- ↑ Ramsden, J. J. (2013). "On the proportion of ortho isomers in the tricresyl phosphates contained in jet oil". Journal of Biological Physics and Chemistry. 13 (2): 69–72. doi:10.4024/03RA13L.jbpc.13.02.
- ↑ Baker, Paul E.; Cole, Toby B.; Cartwright, Megan; Suzuki, Stephanie M.; Thummel, Kenneth E.; Lin, Yvonne S.; Co, Aila L.; Rettie, Allan E.; Kim, Jerry H.; Furlong, Clement E. (2013). "Identifying safer anti-wear triaryl phosphate additives for jet engine lubricants". Chemico-Biological Interactions. 203 (1): 257–264. doi:10.1016/j.cbi.2012.10.005. PMC 3618534.
- ↑ Carletti, Eugenie (2011). "Reaction of cresyl saligenin phosphate, the organophosphorus agent implicated in the aerotoxic syndrome, with human cholinesterases: Mechanistic studies employing kinetics, mass spectrometry and x-ray structure analysis". Chemical Research in Toxicology. 24 (6): 797–808. doi:10.1021/tx100447k. PMC 3118852.
- ↑ "Tri-o-cresyl phosphate". National Library of Medicine HSDB Database. National Institutes of Health.
- ↑ "Tricresyl phosphate (EHC 110, 1990)". www.inchem.org.
- ↑ Baum, Dan. "Annals of Epidemiology: Jake Leg". archives.newyorker.com. The New Yorker.
- ↑ Travers, P. R. (1962). "The Results of Intoxication with Orthocresyl Phosphate Absorbed from Contaminated Cooking Oil, as seen in 4,029 Patients in Morocco". Proceedings of the Royal Society of Medicine. 55 (1): 57–60.
- ↑ Rege, Rekha S.; Singhal, Pushpa R.; Kulkarni, Dinanath V. (1997). Handbook of Indices of Food Quality and Authenticity (1st ed.). Cambridge: Woodhead. ISBN 9781855732995.