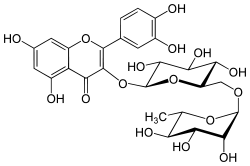
Rutin
 | |
| Names | |
|---|---|
| IUPAC name
2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]-4H-chromen-4-one | |
| Systematic IUPAC name
2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-4H-chromen-4-one | |
| Other names
Rutoside Phytomelin Sophorin Birutan Eldrin Birutan Forte Rutin trihydrate Globularicitrin Violaquercitrin Quercetin rutinoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.287 |
| KEGG | |
PubChem CID |
|
| RTECS number | VM2975000 |
| UNII | |
| |
| |
| Properties | |
| C27H30O16 | |
| Molar mass | 610.52 g·mol−1 |
| Appearance | Solid |
| Melting point | 242 °C (468 °F; 515 K) |
| 12.5 mg/100 mL[1] 13 mg/100mL[2] | |
| Pharmacology | |
| C05CA01 (WHO) | |
| Hazards | |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rutin, also called rutoside, quercetin-3-O-rutinoside and sophorin, is the glycoside combining the flavonol quercetin and the disaccharide rutinose (α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranose). It is a citrus flavonoid found in a wide variety of plants including citrus fruit.
Occurrences
Rutin is one of the phenolic compounds found in the invasive plant species Carpobrotus edulis and contributes to the antibacterial[3] properties of the plant.
Its name comes from the name of Ruta graveolens, a plant that also contains rutin.
Metabolism
The enzyme quercitrinase can be found in Aspergillus flavus.[4] It is an enzyme in the rutin catabolic pathway.[5]
In food
Rutin is a citrus flavonoid glycoside found in many plants including buckwheat,[6] the leaves and petioles of Rheum species, and asparagus. Tartary buckwheat seeds have been found to contain more rutin (about 0.8–1.7% dry weight) than common buckwheat seeds (0.01% dry weight).[6] Rutin is one of the primary flavonols found in 'clingstone' peaches.[7] It is also found in green tea infusions.[8]
Approximate rutin content per 100g of selected foods:[9]
332 mg Capers, spice
45 mg Olive [Black], raw
36 mg Buckwheat, whole grain flour
23 mg Asparagus, raw
19 mg Black raspberry, raw
11 mg Red raspberry, raw
9 mg Buckwheat, groats, thermally treated
6 mg Buckwheat, refined flour
6 mg Greencurrant
6 mg Plum, fresh
5 mg Blackcurrant, raw
4 mg Blackberry, raw
3 mg Tomato [Cherry], whole, raw
2 mg prune
2 mg Fenugreek, fresh
2 mg Marjoram, dried
2 mg Tea [Black], infusion
1 mg Grape, raisin
1 mg Zucchini, raw
1 mg Apricot, raw
1 mg Tea [Green], infusion
0 mg apple
0 mg redcurrant
0 mg Grape [Green]
0 mg Tomato, whole, raw
Research
Rutin (rutoside or rutinoside)[10] and other dietary flavonols are under preliminary clinical research for their potential biological effects, such as in reducing post-thrombotic syndrome, venous insufficiency, or endothelial dysfunction, but there was no high-quality evidence for their safe and effective uses as of 2016.[10][11][12] As a flavonol among similar flavonoids, rutin has low bioavailability due to poor absorption, high metabolism, and rapid excretion that collectively make its potential for use as a therapeutic agent limited.[10]
References
- ↑ Merck Index, 12th Edition, 8456
- ↑ Krewson CF, Naghski J (Nov 1952). "Some physical properties of rutin". Journal of the American Pharmaceutical Association. American Pharmaceutical Association. 41 (11): 582–7. doi:10.1002/jps.3030411106. PMID 12999623.
- ↑ van der Watt E, Pretorius JC (2001). "Purification and identification of active antibacterial components in Carpobrotusedulis L.". Journal of Ethnopharmacology. 76 (1): 87–91. doi:10.1016/S0378-8741(01)00197-0. PMID 11378287.
- ↑ quercitrinase on www.brenda-enzymes.org
- ↑ Tranchimand S, Brouant P, Iacazio G (Nov 2010). "The rutin catabolic pathway with special emphasis on quercetinase". Biodegradation. 21 (6): 833–59. doi:10.1007/s10532-010-9359-7. PMID 20419500.
- 1 2 Kreft S, Knapp M, Kreft I (Nov 1999). "Extraction of rutin from buckwheat (Fagopyrum esculentumMoench) seeds and determination by capillary electrophoresis". Journal of Agricultural and Food Chemistry. 47 (11): 4649–52. doi:10.1021/jf990186p. PMID 10552865.
- ↑ Chang S, Tan C, Frankel EN, Barrett DM (Feb 2000). "Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars". Journal of Agricultural and Food Chemistry. 48 (2): 147–51. doi:10.1021/jf9904564. PMID 10691607.
- ↑ Malagutti AR, Zuin V, Cavalheiro ÉT, Henrique Mazo L (2006). "Determination of Rutin in Green Tea Infusions Using Square‐Wave Voltammetry with a Rigid Carbon‐Polyurethane Composite Electrode". Electroanalysis. 18 (10): 1028–1034. doi:10.1002/elan.200603496.
- ↑ "foods in which the polyphenol Quercetin 3-O-rutinoside is found". Phenol-Explorer v 3.6. June 2015.
- 1 2 3 "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, Oregon. November 2015. Retrieved 25 February 2018.
- ↑ Morling, J. R; Yeoh, S. E; Kolbach, D. N (2015). "Rutosides for treatment of post-thrombotic syndrome". Cochrane Database of Systematic Reviews (9): CD005625. doi:10.1002/14651858.CD005625.pub3. PMID 26376212.
- ↑ Martinez-Zapata, M. J; Vernooij, R. W; Uriona Tuma, S. M; Stein, A. T; Moreno, R. M; Vargas, E; Capellà, D; Bonfill Cosp, X (2016). "Phlebotonics for venous insufficiency". Cochrane Database of Systematic Reviews. 4: CD003229. doi:10.1002/14651858.CD003229.pub3. PMID 27048768.
External links

