Rovalpituzumab tesirine
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | DLL3 |
| Clinical data | |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
| Formula | C6416H9894N1698O2028S46 (non-glycosylated) |
Rovalpituzumab tesirine (Rova-T) is an experimental antibody-drug conjugate targeting the protein DLL3 on tumor cells.[1] It was originally developed by Stemcentrx and was purchased by AbbVie[2]. It is being tested for use in small-cell lung cancer.[3]
Development
A phase III trial is evaluating the drug as a maintenance therapy after chemotherapy for small cell lung cancer.[4] A phase II trial is using the drug as a third-line treatment for relapsed or refractory lung cancer.[5]
Chemical structure
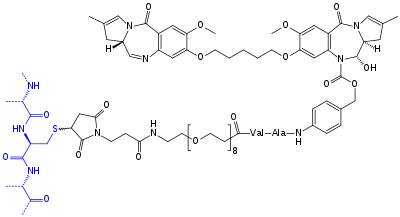
Chemical structure of "tesirine" (drawn in black). It consists of a pyrrolobenzodiazepine type dimer (top), which is the actual anti-cancer agent, a Val–Ala structure that can be cleaved by an enzyme to detach the anti-cancer agent from the antibody, a polyethylene glycol spacer, and a maleimide linker which is attached to a cysteine in the antibody's (rovalpituzumab's) peptide backbone, drawn blue. Each rovalpituzumab molecule has an average of two such attachments.[6]
See also
- Vadastuximab talirine, with a similar cytotoxin
References
- ↑ "Statement On A Nonproprietary Name Adopted By The USAN Council: USAN (de-144) Rovalpituzumab" (PDF). Searchusan.ama-assn.org. Retrieved 2017-05-23.
- ↑ http://bciq.biocentury.com/products/rova-t
- ↑ Alternative Names: Rova-T; SC16LD6.5. "Rovalpituzumab tesirine - AdisInsight". Adisinsight.springer.com. Retrieved 2017-05-22.
- ↑ "A Study of Rovalpituzumab Tesirine as Maintenance Therapy Following First-Line Platinum-Based Chemotherapy in Participants With Extensive Stage Small Cell Lung Cancer (MERU) - Full Text View". ClinicalTrials.gov. Retrieved 2017-05-22.
- ↑
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended INN: List 75" (PDF). WHO Drug Information. World Health Organization. 30 (1): 151. 2016.