Dimethoate
 | |
| Names | |
|---|---|
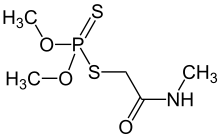
| IUPAC name
O,O-dimethyl S-[2-(methylamino)-2-oxoethyl] dithiophosphate | |
| Other names
O,O-dimethyl S-methylcarbamoylmethyl phosphorodithioate Phosphorodithioic acid, O,O-Dimethyl S-(2-(methylamino)-2-oxoethylyl)ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.437 |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C5H12NO3PS2 | |
| Molar mass | 229.26 g/mol |
| Appearance | Grey-white crystalline solid |
| Density | 1.3 g/cm3, solid |
| Melting point | 43 to 45 °C (109 to 113 °F; 316 to 318 K) |
| Boiling point | 117 °C (243 °F; 390 K) at 10 Pa |
| 2.5 g/100 ml | |
| Hazards | |
| Main hazards | Highly toxic |
| Safety data sheet | External MSDS |
| GHS pictograms |  |
| H302 - H312[1] | |
| P280[1] | |
| Flash point | 107 °C (225 °F; 380 K) |
| Related compounds | |
Related organophosphates |
malathion |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethoate is a widely used organophosphate insecticide and acaricide. It was patented and introduced in the 1950s by American Cyanamid. Like other organophosphates, dimethoate is an acetylcholinesterase inhibitor which disables cholinesterase, an enzyme essential for central nervous system function. It acts both by contact and through ingestion. It is readily absorbed and distributed throughout plant tissues, and is degraded relatively rapidly.[2]
Trade names
References
- 1 2 3 Sigma-Aldrich Co., Dimethoate. Retrieved on 2013-07-20.
- ↑ Dauterman, W. C.; Viado, G. B.; Casida, J. E.; O'Brien, R. D. (1960). "Insecticide Residues, Persistence of Dimethoate and Metabolites Following Foliar Application to Plants". Journal of Agricultural and Food Chemistry. 8 (2): 115–9. doi:10.1021/jf60108a013.
- ↑ Padmasheela, N. C.; Delvi, M. R. (2004). "Effect of Dimethoate (Rogor 30% EC) on the brain neurosecretory cells of third instar grubs of Oryctes rhinoceros L. (Coleoptera : Scarabaeidae)". Journal of Environmental Biology. 25 (4): 451–5. PMID 15907075.
- ↑ https://www.ravensdown.co.nz/products/agrochemicals/rogor%5Bfull+citation+needed%5D
External links
- EPA Report on Dimethoate(dead link)
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.