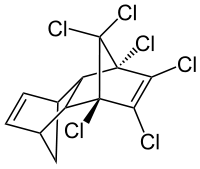
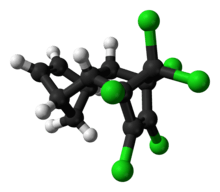
Aldrin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,2,3,4,10,10-Hexachloro- | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.652 |
| EC Number | 206-215-8 |
| KEGG | |
PubChem CID |
|
| RTECS number | IO2100000 |
| UNII | |
| UN number | 2762, 2761 |
| |
| |
| Properties | |
| C12H8Cl6 | |
| Molar mass | 364.90 g·mol−1 |
| Appearance | colorless solid |
| Density | 1.60 g/mL[1] |
| Melting point | 104 °C (219 °F; 377 K) |
| slightly soluble (0.003%)[1] | |
| Vapor pressure | 7.5 × 10−5 mmHg @ 20 °C |
| Hazards | |
| Main hazards | potential occupational carcinogen[1] |
| GHS pictograms |    |
| GHS signal word | Danger |
| H300, H301, H301, H310, H311, H351, H372, H400, H410 | |
| P201, P202, P260, P262, P264, P270, P273, P280, P281, P301+310, P302+350, P302+352, P308+313, P310, P312, P314, P321, P322, P330, P361, P363, P391, P405, P501 | |
| NFPA 704 | |
| Flash point | 66 °C (151 °F; 339 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
50 mg/kg (rabbit, oral) 33 mg/kg (guinea pig, oral) 39 mg/kg (rat, oral) 44 mg/kg (mouse, oral)[2] |
LCLo (lowest published) |
5.8 mg/m3 (rat, 4 hr)[2] |
| US health exposure limits (NIOSH): | |
PEL (Permissible) |
TWA 0.25 mg/m3 [skin][1] |
REL (Recommended) |
Ca TWA 0.25 mg/m3 [skin][1] |
IDLH (Immediate danger) |
25 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aldrin is an organochlorine insecticide that was widely used until the 1990s, when it was banned in most countries. It is a colourless solid. Before the ban, it was heavily used as a pesticide to treat seed and soil. Aldrin and related "cyclodiene" pesticides (a term for pesticides derived from Hexachlorocyclopentadiene) became notorious as persistent organic pollutants.[3]
Production
Aldrin is produced by combining hexachlorocyclopentadiene with norbornadiene in a Diels-Alder reaction to give the adduct.[4]
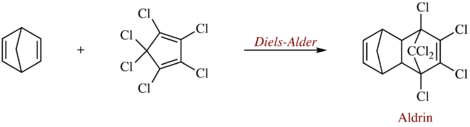
Similarly, an isomer of aldrin, known as isodrin, is produced by reaction of hexachloronobornadiene with cyclopentadiene.[5]
Aldrin is named after the German chemist Kurt Alder, one of the coinventors of this kind of reaction. An estimated 270 million kilograms of aldrin and related cyclodiene pesticides were produced between 1946 and 1976.
In soil, on plant surfaces, or in the digestive tracts of insects, aldrin oxidizes to the epoxide dieldrin, which is more strongly insecticidal.
Environmental impact and regulation
Like related polychlorinated pesticides, aldrin is highly lipophilic. Its solubility in water is only 0.027 mg/L, which exacerbates its persistence in the environment. It was banned by the Stockholm Convention on Persistent Organic Pollutants. In the U.S., aldrin was cancelled in 1974. The substance is banned from use for plant protection by the EU.[6]
Safety and environmental aspects
Aldrin has rat LD50 of 39 to 60 mg/kg (oral in rats). For fish however, it is extremely toxic, with an LC50 of 0.006 – 0.01 for trout and bluegill.[3]
In the US, aldrin is considered a potential occupational carcinogen by the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health; these agencies have set an occupational exposure limit for dermal exposures at 0.25 mg/m3 over an eight-hour time-weighted average.[7] Further, an IDLH limit has been set at 25 mg/m3, based on acute toxicity data in humans to which subjects reacted with convulsions within 20 minutes of exposure.[8]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[9]
References
- 1 2 3 4 5 6 7 8 "NIOSH Pocket Guide to Chemical Hazards #0016". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 "Aldrin". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Robert L. Metcalf "Insect Control" in Ullmann’s Encyclopedia of Industrial Chemistry" Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a14_263
- ↑ Jubb, A. H. (1975). Basic Organic Chemistry, Part 5 Industrial products. London: Wiley. ISBN 0-471-85014-4.
- ↑ Bird, C. W.; Cookson, R. C.; Crundwell, E. (1961). "946. Cyclisations and rearrangements in the isodrin?aldrin series". Journal of the Chemical Society (Resumed): 4809. doi:10.1039/JR9610004809.
- ↑ Chemicals Regulation Directorate. "Banned and Non-Authorised Pesticides in the United Kingdom". Retrieved 1 December 2009.
- ↑ Centers for Disease Control and Prevention (4 April 2011). "Aldrin". NIOSH Pocket Guide to Chemical Hazards. Retrieved 13 November 2013.
- ↑ Centers for Disease Control and Prevention (May 1994). "Aldrin". Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs). Retrieved 13 November 2013.
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Retrieved October 29, 2011.
