Pyroglutamic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
5-Oxoproline | |
| Systematic IUPAC name
5-Oxopyrrolidine-2-carboxylic acid | |
| Other names
2-Pyrrolidone-5-carboxylic acid Pidolic acid 5-Oxo-proline | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | B01555 |
| Abbreviations | Glp |
| 82134 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.021.578 |
| EC Number | 205-748-3 |
| 1473408 | |
| KEGG | |
| MeSH | Pyrrolidonecarboxylic+acid |
PubChem CID |
|
| RTECS number | TW3710000 |
| UNII | |
| |
| |
| Properties | |
| C5H7NO3 | |
| Molar mass | 129.12 g·mol−1 |
| Melting point | 184 °C (363 °F; 457 K) |
| log P | -0.89 |
| Acidity (pKa) | -1.76, 3.48, 12.76 |
| Basicity (pKb) | 15.76, 10.52, 1.24 |
| Isoelectric point | 0.94 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pyroglutamic acid (also known as PCA, 5-oxoproline, pidolic acid, or pyroglutamate for its basic form) is a ubiquitous but little studied natural amino acid derivative in which the free amino group of glutamic acid or glutamine cyclizes to form a lactam.[1]
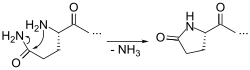
It is a metabolite in the glutathione cycle that is converted to glutamate by 5-oxoprolinase. Pyroglutamate is found in many proteins including bacteriorhodopsin. N-terminal glutamic acid and glutamine residues can spontaneously cyclize to become pyroglutamate, or enzymatically converted by glutaminyl cyclases.[2] This is one of several forms of blocked N-termini which present a problem for N-terminal sequencing using Edman chemistry, which requires a free primary amino group not present in pyroglutamic acid. The enzyme pyroglutamate aminopeptidase can restore a free N-terminus by cleaving off the pyroglutamate residue.[3]
Pyroglutamic acid, also known as pidolic acid, exists as two distinct enantiomers:
- (2R) or D which happens to be (+) or d
- (2S) or L which happens to be (–) or l
Metabolism
As first discovered in 1882, pyroglutamic acid can be formed by heating glutamic acid at 180 °C, which results in the loss of a molecule of water. In living cells, it is derived from glutathione through the action of an enzyme, γ-glutamyl cyclotransferase.[1] It may function to store glutamate, but also acts to oppose the action of glutamate including in the brain.[1] It also acts on the cholinergic system in the brain,[4] and Amyloid β containing pyroglutamic acid is increased in Alzheimer's disease and may be involved in the disease process.[5] Levels of pyroglutamic acid in the blood, called 5-oxoprolinuria, can increase following paracetamol overdose or in inborn error of metabolisms, causing an increased level of acidity called a high anion gap metabolic acidosis.[1][6]
Uses
The sodium salt of pyroglutamic acid — known either as sodium pyroglutamate, sodium PCA, or sodium pidolate — is used for dry skin and hair products, as it is a humectant. It has low toxicity and is not a skin irritant, but its use in products is limited by a high price.[7][8]
L-pyroglutamic acid is sold online as a dietary supplement.[9][10]
Magnesium pidolate, the magnesium salt of pyroglutamic acid, is a mineral supplement.
References
- 1 2 3 4 Kumar, Akhilesh; Bachhawat, Anand K. (January 25, 2012). "Pyroglutamic acid: throwing light on a lightly studied metabolite" (PDF). 102 (2): 208.
- ↑ Schilling, S.; Wasternack, C.; Demuth, H.U. (2008), "Glutaminyl cyclases from animals and plants: a case of functionally convergent protein evolution", Biol. Chem., 389 (8): 983–91, doi:10.1515/BC.2008.111, PMID 18979624 .
- ↑ Podell, David N.; Abraham, George N. (1978), "A technique for the removal of pyroglutamic acid from the amino terminus of proteins using calf liver pyroglutamate amino peptidase", Biochem. Biophys. Res. Commun., 81 (1): 176–85, doi:10.1016/0006-291X(78)91646-7, PMID 26343 .
- ↑ Pepeu, Giancarlo; Spignoli, Giacomo (1989). "Nootropic drugs and brain cholinergic mechanisms". Prog Neuropsychopharmacol Biol Psychiatry. 13 (Supplement 1): S77–88. doi:10.1016/0278-5846(89)90112-7.
- ↑ Jawhar, S; Wirths, O; Bayer, TA (November 11, 2011). "Pyroglutamate amyloid-β (Aβ): a hatchet man in Alzheimer disease". J Biol Chem. 286 (45): 38825–32. doi:10.1074/jbc.R111.288308. PMC 3234707. PMID 21965666.
- ↑ Liss, DB; Paden, MS; Schwarz, ES; Mullins, ME (November 2013). "What is the clinical significance of 5-oxoproline (pyroglutamic acid) in high anion gap metabolic acidosis following paracetamol (acetaminophen) exposure?". Clin Toxicol. 51 (9): 817–27. doi:10.3109/15563650.2013.844822.
- ↑ "Hydromol® (Alliance)". British National Formulary. Retrieved December 5, 2015.
- ↑ Jungermann, Eric; Sonntag, Norman O.V (1991-07-19). "Alternatives to Glycerine". In Eric Jungermann, Norman O.V. Sonnta. Glycerine: A Key Cosmetic Ingredient. p. 424. ISBN 9780824784652.
- ↑ DellaVecchia, Matthew J. (December 2013). "Inaccurate Serelaxin Chemical Structure". Pharmacy and Therapeutics. 38 (12): 763. PMC 3875272. PMID 24391398.
- ↑ McDougall, Jr., Graham J.; Austin-Wells, Vonnette; Zimmerman, Teena (December 2005). "Utility of Nutraceutical Products Marketed for Cognitive and Memory Enhancement". J Holist Nurs. 23 (4): 415–433. doi:10.1177/0898010105280097. PMC 2398696. PMID 16251490. (table 1)