Plankton

Plankton (singular plankter) are the diverse collection of organisms that live in large bodies of water and are unable to swim against a current.[1] They provide a crucial source of food to many large aquatic organisms, such as fish and whales.
These organisms include bacteria, archaea, algae, protozoa and drifting or floating animals that inhabit—for example—the pelagic zone of oceans, seas, or bodies of fresh water. Essentially, plankton are defined by their ecological niche rather than any phylogenetic or taxonomic classification.
Though many planktonic species are microscopic in size, plankton includes organisms over a wide range of sizes, including large organisms such as jellyfish.[2] Technically the term does not include organisms on the surface of the water, which are called pleuston—or those that swim actively in the water, which are called nekton.
Terminology
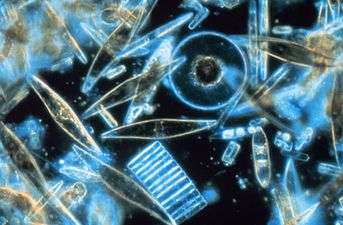
The name plankton is derived from the Greek adjective πλαγκτός (planktos), meaning errant, and by extension, wanderer or drifter,[3] and was coined by Victor Hensen in 1887.[4][5] While some forms are capable of independent movement and can swim hundreds of meters vertically in a single day (a behavior called diel vertical migration), their horizontal position is primarily determined by the surrounding water movement, and plankton typically flow with ocean currents. This is in contrast to nekton organisms, such as fish, squid and marine mammals, which can swim against the ambient flow and control their position in the environment.
Within the plankton, holoplankton spend their entire life cycle as plankton (e.g. most algae, copepods, salps, and some jellyfish). By contrast, meroplankton are only planktic for part of their lives (usually the larval stage), and then graduate to either a nektic (swimming) or benthic (sea floor) existence. Examples of meroplankton include the larvae of sea urchins, starfish, crustaceans, marine worms, and most fish.[6]
The amount and distribution of plankton depends on available nutrients, the state of water and a large amount of other plankton.[7]
The study of plankton is termed planktology and a planktonic individual is referred to as a plankter.[8] The adjective planktonic is widely used in both the scientific and popular literature, and is a generally accepted term. However, from the standpoint of prescriptive grammar, the less commonly used planktic is more strictly the correct adjective. When deriving English words from their Greek or Latin roots, the gender-specific ending (in this case "-on," which indicates the word is neuter) is normally dropped, using only the root of the word in the derivation.[9]
Trophic groups

Plankton are primarily divided into broad functional (or trophic level) groups:
- Phytoplankton (from Greek phyton, or plant), autotrophic prokaryotic or eukaryotic algae that live near the water surface where there is sufficient light to support photosynthesis. Among the more important groups are the diatoms, cyanobacteria, dinoflagellates and coccolithophores.
- Zooplankton (from Greek zoon, or animal), small protozoans or metazoans (e.g. crustaceans and other animals) that feed on other plankton. Some of the eggs and larvae of larger nektonic animals, such as fish, crustaceans, and annelids, are included here.
- Bacterioplankton, bacteria and archaea, which play an important role in remineralising organic material down the water column (note that prokaryotic phytoplankton are also bacterioplankton).
- Mycoplankton, fungi and fungus-like organisms, which, like bacterioplankton, are also significant in remineralisation and nutrient cycling.[10]
This scheme divides the plankton community into broad producer, consumer and recycler groups. However, determining the trophic level of many plankton is not always straightforward. For example, although most dinoflagellates are either photosynthetic producers or heterotrophic consumers, many species perform both roles. In this mixed trophic strategy — known as mixotrophy — organisms act as both producers and consumers, either at the same time or switching between modes of nutrition in response to ambient conditions. For instance, relying on photosynthesis for growth when nutrients and light are abundant, but switching to predation when growing conditions are poor. Recognition of the importance of mixotrophy as an ecological strategy is increasing,[11] as well as the wider role this may play in marine biogeochemistry.[12]
Size groups

Plankton are also often described in terms of size.[13] Usually the following divisions are used:
Group Size range
(ESD)Examples Megaplankton > 20 cm metazoans; e.g. jellyfish; ctenophores; salps and pyrosomes (pelagic Tunicata); Cephalopoda; Amphipoda Macroplankton 2→20 cm metazoans; e.g. Pteropods; Chaetognaths; Euphausiacea (krill); Medusae; ctenophores; salps, doliolids and pyrosomes (pelagic Tunicata); Cephalopoda; Janthinidae (one family of gastropods); Amphipoda Mesoplankton 0.2→20 mm metazoans; e.g. copepods; Medusae; Cladocera; Ostracoda; Chaetognaths; Pteropods; Tunicata Microplankton 20→200 µm large eukaryotic protists; most phytoplankton; Protozoa Foraminifera; tintinnids; other ciliates; Rotifera; juvenile metazoans - Crustacea (copepod nauplii) Nanoplankton 2→20 µm small eukaryotic protists; Small Diatoms; Small Flagellates; Pyrrophyta; Chrysophyta; Chlorophyta; Xanthophyta Picoplankton 0.2→2 µm small eukaryotic protists; bacteria; Chrysophyta Femtoplankton < 0.2 µm marine viruses
However, some of these terms may be used with very different boundaries, especially on the larger end. The existence and importance of nano- and even smaller plankton was only discovered during the 1980s, but they are thought to make up the largest proportion of all plankton in number and diversity.
The microplankton and smaller groups are microorganisms and operate at low Reynolds numbers, where the viscosity of water is much more important than its mass or inertia. [14]
Distribution
Plankton inhabit oceans, seas, lakes, ponds. Local abundance varies horizontally, vertically and seasonally. The primary cause of this variability is the availability of light. All plankton ecosystems are driven by the input of solar energy (but see chemosynthesis), confining primary production to surface waters, and to geographical regions and seasons having abundant light.
A secondary variable is nutrient availability. Although large areas of the tropical and sub-tropical oceans have abundant light, they experience relatively low primary production because they offer limited nutrients such as nitrate, phosphate and silicate. This results from large-scale ocean circulation and water column stratification. In such regions, primary production usually occurs at greater depth, although at a reduced level (because of reduced light).
Despite significant macronutrient concentrations, some ocean regions are unproductive (so-called HNLC regions).[15] The micronutrient iron is deficient in these regions, and adding it can lead to the formation of phytoplankton blooms.[16] Iron primarily reaches the ocean through the deposition of dust on the sea surface. Paradoxically, oceanic areas adjacent to unproductive, arid land thus typically have abundant phytoplankton (e.g., the eastern Atlantic Ocean, where trade winds bring dust from the Sahara Desert in north Africa).
While plankton are most abundant in surface waters, they live throughout the water column. At depths where no primary production occurs, zooplankton and bacterioplankton instead consume organic material sinking from more productive surface waters above. This flux of sinking material, so-called marine snow, can be especially high following the termination of spring blooms.
Ecological significance
Food chain
Aside from representing the bottom few levels of a food chain that supports commercially important fisheries, plankton ecosystems play a role in the biogeochemical cycles of many important chemical elements, including the ocean's carbon cycle.[17]
Carbon cycle
Primarily by grazing on phytoplankton, zooplankton provide carbon to the planktic foodweb, either respiring it to provide metabolic energy, or upon death as biomass or detritus. Organic material tends to be denser than seawater, so it sinks into open ocean ecosystems away from the coastlines, transporting carbon along with it. This process, called the biological pump, is one reason that oceans constitute the largest carbon sink on Earth. However, it has been shown to be influenced by increments of temperature.[18][19][20][21]
It might be possible to increase the ocean's uptake of carbon dioxide (CO
2) generated through human activities by increasing plankton production through seeding, primarily with the micronutrient iron. However, this technique may not be practical at a large scale. Ocean oxygen depletion and resultant methane production (caused by the excess production remineralising at depth) is one potential drawback.[22][23]
Oxygen production
Phytoplankton absorb energy from the Sun and nutrients from the water to produce their own nourishment or energy. In the process of photosynthesis, phytoplankton release molecular oxygen (O
2) into the water as a waste biproduct. It is estimated that about 50% of the world's oxygen is produced via phytoplankton photosynthesis.[24] The rest is produced via photosynthesis on land by plants.[24] Furthermore, phytoplankton photosynthesis has controlled the atmospheric CO
2/O
2 balance since the early Precambrian Eon.[25]
Biomass variability
The growth of phytoplankton populations is dependent on light levels and nutrient availability. The chief factor limiting growth varies from region to region in the world's oceans. On a broad scale, growth of phytoplankton in the oligotrophic tropical and subtropical gyres is generally limited by nutrient supply, while light often limits phytoplankton growth in subarctic gyres. Environmental variability at multiple scales influences the nutrient and light available for phytoplankton, and as these organisms form the base of the marine food web, this variability in phytoplankton growth influences higher trophic levels. For example, at interannual scales phytoplankton levels temporarily plummet during El Niño periods, influencing populations of zooplankton, fishes, sea birds, and marine mammals.
The effects of anthropogenic warming on the global population of phytoplankton is an area of active research. Changes in the vertical stratification of the water column, the rate of temperature-dependent biological reactions, and the atmospheric supply of nutrients are expected to have important impacts on future phytoplankton productivity.[26] Additionally, changes in the mortality of phytoplankton due to rates of zooplankton grazing may be significant.
Freshly hatched fish larvae are also plankton for a few days, as long as it takes before they can swim against currents.
 An amphipod: an exoskeletoned animal with curved body, with two long and two short antennae.
An amphipod: an exoskeletoned animal with curved body, with two long and two short antennae.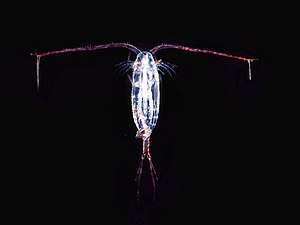
.jpg) Antarctic krill, probably the largest biomass of a single species on the planet
Antarctic krill, probably the largest biomass of a single species on the planet Northern krill: the mid gut is red. It feeds on zooplankton
Northern krill: the mid gut is red. It feeds on zooplankton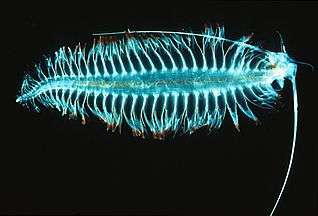 Tomopteris is a genus of marine planktonic polychaete
Tomopteris is a genus of marine planktonic polychaete Siphonophora – the "conveyor belt" of the upgrowing larvae and the ovarium can be seen
Siphonophora – the "conveyor belt" of the upgrowing larvae and the ovarium can be seen Sea foam can be produced by plankton, photo of many, differently sized bubbles with image of photographer
Sea foam can be produced by plankton, photo of many, differently sized bubbles with image of photographer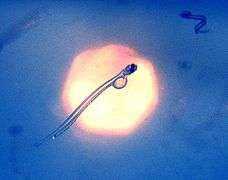 Herring larva imaged in situ in the typical oblique swimming position with the remains of the yolk and the long gut visible in the transparent animal
Herring larva imaged in situ in the typical oblique swimming position with the remains of the yolk and the long gut visible in the transparent animal Eel larva drifting with the gulf stream
Eel larva drifting with the gulf stream Icefish larvae from Antarctica have no haemoglobin
Icefish larvae from Antarctica have no haemoglobin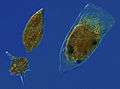 Microzooplankton, the major grazers of the plankton: Dinoflagellates (spindle-shaped 'Gyrodinium', spiny-globe 'Protoperidinium') and a tintinnid ciliate (hairy-topped cell in a shell, 'Favella'). From the Thau Lagoon of Sète, France
Microzooplankton, the major grazers of the plankton: Dinoflagellates (spindle-shaped 'Gyrodinium', spiny-globe 'Protoperidinium') and a tintinnid ciliate (hairy-topped cell in a shell, 'Favella'). From the Thau Lagoon of Sète, France
Importance to fish
Zooplankton are the initial prey item for almost all fish larvae as they switch from their yolk sacs to external feeding. Fish rely on the density and distribution of zooplankton to match that of new larvae, which can otherwise starve. Natural factors (e.g., current variations) and man-made factors (e.g. river dams) can strongly affect zooplankton, which can in turn strongly affect larval survival, and therefore breeding success.
The importance of both phytoplankton and zooplankton is also well-recognized in extensive and semi-intensive pond fish farming. Plankton population based pond management strategies for fish rearing have been practised by traditional fish farmers for decades, illustrating the importance of plankton even in man-made environments.
See also
References
- ↑ Lalli, C.; Parsons, T. (1993). Biological Oceanography: An Introduction. Butterworth-Heinemann. ISBN 0 7506 3384 0.
- ↑ John Dolan (November 2012). "Microzooplankton: the microscopic (micro) animals (zoo) of the plankton" (PDF).
- ↑ Thurman, H. V. (1997). Introductory Oceanography. New Jersey, USA: Prentice Hall College. ISBN 978-0-13-262072-7.
- ↑ Hensen, V. 1887. Uber die Bestimmung des Planktons oder des im Meere treibenden Materials an Pflanzen und Thieren. V. Bericht der Commission zur Wissenschaftlichen Untersuchung der Deutschen Meere, Jahrgang 12-16, p. 1-108, .
- ↑ "Online Etymology Dictionary". etymonline.com.
- ↑ Karleskint, George; Turner, Richard; Small, James (2013). "Chapter 17: The Open Sea". Introduction to Marine Biology (4th ed.). Brooks/Cole. ISBN 978-1-133-36446-7.
- ↑ Agrawai, Anju; Gopnal, Krishna (2013). Biomonitoring of Water and Waste Water. Springer India 2013. ISBN 9788132208648. Retrieved April 2, 2018.
- ↑ "plankter - marine biology". Encyclopædia Britannica.
- ↑ Emiliani, C. (1991). "Planktic/Planktonic, Nektic/Nektonic, Benthic/Benthonic". Journal of Paleontology. 65 (2): 329. JSTOR 1305769.
- ↑ Wang, G., Wang, X., Liu, X., & Li, Q. (2012). Diversity and biogeochemical function of planktonic fungi in the ocean. In: C. Raghukumar (ed.), Biology of marine fungi. Springer Berlin Heidelberg, p. 71-88, .
- ↑ Hartmann, M.; Grob, C.; Tarran, G.A.; Martin, A.P.; Burkill, P.H.; Scanlan, D.J.; Zubkov, M.V. (2012). "Mixotrophic basis of Atlantic oligotrophic ecosystems". Proc. Natl. Acad. Sci. USA. 109 (15): 5756–5760. Bibcode:2012PNAS..109.5756H. doi:10.1073/pnas.1118179109. PMC 3326507. PMID 22451938. Retrieved 28 April 2017.
- ↑ Ward, B.A.; Follows, M.J. (2016). "Marine mixotrophy increases trophic transfer efficiency, mean organism size, and vertical carbon flux". Proc. Natl. Acad. Sci. USA. 113 (11): 2958–2963. Bibcode:2016PNAS..113.2958W. doi:10.1073/pnas.1517118113. PMC 4801304. PMID 26831076. Retrieved 28 April 2017.
- ↑ Omori, M.; Ikeda, T. (1992). Methods in Marine Zooplankton Ecology. Malabar, USA: Krieger Publishing Company. ISBN 978-0-89464-653-9.
- ↑ Dusenbery, David B. (2009). Living at micro scale: the unexpected physics of being small. Cambridge: Harvard University Press. ISBN 978-0-674-03116-6.
- ↑ Martin, J. H.; Fitzwater, S. E. (1988). "Iron-deficiency limits phytoplankton growth in the Northeast Pacific Subarctic". Nature. 331 (6154): 341–343. Bibcode:1988Natur.331..341M. doi:10.1038/331341a0.
- ↑ Boyd, P.W.; et al. (2000). "A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by fertilization". Nature. 407 (6805 http://tass.ru/en/non-political/745635): 695–702. doi:10.1038/35037500. PMID 11048709.
- ↑ Falkowski, Paul G. (1994). "The role of phytoplankton photosynthesis in global biogeochemical cycles" (PDF). Photosyntheis Research. 39 (3): 235–258. doi:10.1007/BF00014586. PMID 24311124.
- ↑ Sarmento, H.; Montoya, JM.; Vázquez-Domínguez, E.; Vaqué, D.; Gasol, JM. (2010). "Warming effects on marine microbial food web processes: how far can we go when it comes to predictions?". Philosophical Transactions of the Royal Society B: Biological Sciences. 365 (1549): 2137–2149. doi:10.1098/rstb.2010.0045. PMC 2880134. PMID 20513721.
- ↑ Vázquez-Domínguez, E.; Vaqué, D.; Gasol, JM. (2007). "Ocean warming enhances respiration and carbon demand of coastal microbial plankton". Global Change Biology. 13 (7): 1327–1334. Bibcode:2007GCBio..13.1327V. doi:10.1111/j.1365-2486.2007.01377.x. hdl:10261/15731.
- ↑ Vázquez-Domínguez, E.; Vaqué, D.; Gasol, JM. (2012). "Temperature effects on the heterotrophic bacteria, heterotrophic nanoflagellates, and microbial top predators of NW Mediterranean". Aquatic Microbial Ecology. 67 (2): 107–121. doi:10.3354/ame01583.
- ↑ Mazuecos, E.; Arístegui, J.; Vázquez-Domínguez, E.; Ortega-Retuerta, E.; Gasol, JM.; Reche, I. (2012). "Temperature control of microbial respiration and growth efficiency in the mesopelagic zone of the South Atlantic and Indian Oceans". Deep Sea Research Part I: Oceanographic Research Papers. 95 (2): 131–138. doi:10.3354/ame01583.
- ↑ Chisholm, S.W.; et al. (2001). "Dis-crediting ocean fertilization". Science. 294 (5541): 309–310. doi:10.1126/science.1065349. PMID 11598285.
- ↑ Aumont, O.; Bopp, L. (2006). "Globalizing results from ocean in situ iron fertilization studies". Global Biogeochemical Cycles. 20 (2): GB2017. Bibcode:2006GBioC..20.2017A. doi:10.1029/2005GB002591.
- 1 2 Roach, John (June 7, 2004). "Source of Half Earth's Oxygen Gets Little Credit". National Geographic News. Retrieved 2016-04-04.
- ↑ Tappan, Helen (April 1968). "Primary production, isotopes, extinctions and the atmosphere". Palaeogeography, Palaeoclimatology, Palaeoecology. 4 (3): 187–210. Bibcode:1968PPP.....4..187T. doi:10.1016/0031-0182(68)90047-3. Retrieved 2016-04-04.
- ↑ Steinacher, M., et al. (2010), Projected 21st century decrease in marine productivity: a multi-model analysis, Biogeosciences, 7, 979-1005
Further reading
- Kirby, Richard R. (2010). Ocean Drifters: A Secret World Beneath the Waves. Studio Cactus Ltd, UK. ISBN 978-1-904239-10-9.
- Dusenbery, David B. (2009). Living at Micro Scale: The Unexpected Physics of Being Small. Harvard University Press, Cambridge, Massachusetts ISBN 978-0-674-03116-6.
- Kiørboe, Thomas (2008). A Mechanistic Approach to Plankton Ecology. Princeton University Press, Princeton, N.J. ISBN 978-0-691-13422-2.
- Dolan, J.R., Agatha, S., Coats, D.W., Montagnes, D.J.S., Stocker, D.K., eds. (2013).Biology and Ecology of Tintinnid Ciliates: Models for Marine Plankton. Wiley-Blackwell, Oxford, UK ISBN 978-0-470-67151-1.
External links
| Look up plankton in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Plankton. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Plankton. |
- Ocean Drifters – Short film narrated by David Attenborough about the varied roles of plankton
- Plankton Chronicles – Short documentary films and photos
- COPEPOD: The Global Plankton Database – Global coverage database of zooplankton biomass and abundance data
- Plankton*Net – Taxonomic database of images of plankton species
- Guide to the marine zooplankton of south eastern Australia – Tasmanian Aquaculture and Fisheries Institute
- Sir Alister Hardy Foundation for Ocean Science – Continuous Plankton Recorder Survey
- Australian Continuous Plankton Recorder Project – Integrated Marine Observing System
- Sea Drifters – BBC Audio slideshow
- – Images of planktonic microorganisms
- Plankton, planktic, planktonic – Essays on nomenclature
- Journal of Plankton Research – Scientific periodical devoted to plankton

