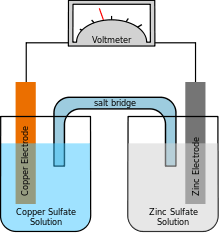
Metal–air electrochemical cell
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte. [1][2] During discharging of a metal–air electrochemical cell, an oxygen reduction reaction occurs in the ambient air cathode while the metal anode is oxidized. The specific capacity and energy density of metal–air electrochemical cells is higher than that of lithium-ion batteries, making them a prime candidate for use in electric vehicles. However, complications associated with the metal anodes, catalysts, and electrolytes have hindered development and implementation of metal–air batteries.[3][4]
Types
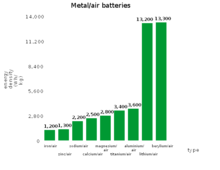
| Metal–air battery | Theoretical specific energy, Wh/kg (including oxygen) |
Theoretical specific energy, Wh/kg (excluding oxygen) |
Calculated open-circuit voltage, V |
|---|---|---|---|
| Aluminium–air | 4300[5] | 8140[6] | 1.2 |
| Germanium–air | 1480 | 7850 | 1 |
| Calcium–air | 2990 | 4180 | 3.12 |
| Iron–air | 1431 | 2044 | 1.3 |
| Lithium–air | 5210 | 11140 | 2.91 |
| Magnesium–air | 2789 | 6462 | 2.93 |
| Potassium–air | 935[7][8] | 1700[Note 1] | 2.48[7][8] |
| Sodium–air | 1677 | 2260 | 2.3[9][10] |
| Silicon–air | 4217 | 9036 | 1.6[11] |
| Tin–air at 1000 K [12] | 860 | 6250 | 0.95 |
| Zinc–air | 1090 | 1350 | 1.65 |
Lithium–air
The remarkably high energy density of lithium metal (up to 3458 Wh/kg) inspired the design of lithium–air batteries. A lithium–air battery consists of a solid lithium electrode, an electrolyte surrounding this electrode, and an ambient air electrode containing oxygen. Current lithium–air batteries can be divided into four subcategories based on the electrolyte used and the subsequent electrochemical cell architecture. These electrolyte categories are aprotic, aqueous, mixed aqueous/aprotic, and solid state, all of which offer their own distinct advantages and disadvantages.[13]. Nonetheless, efficiency of lithium air batteries is still limited by incomplete discharge at the cathode, charging overpotential exceeding discharge overpotential, and component stability.[14] During discharge of lithium–air batteries, the superoxide ion (O2-) formed will react with the electrolyte or other cell components and will prevent the battery from being rechargeable.[15]
Sodium–air
Sodium–air batteries were proposed with the hopes of overcoming the battery instability associated with superoxide in lithium–air batteries. Sodium, with an energy density of 1605 Wh/kg, does not boast as high an energy density as lithium. However, it can form a stable superoxide (NaO2) as opposed to the superoxide undergoing detrimental secondary reactions. Since NaO2 will decompose reversibly to an extent back to the elemental components, this means sodium–air batteries have some intrinsic capacity to be rechargeable.[16] Sodium–air batteries can only function with aprotic, anhydrous electrolytes. When a DMSO electrolyte was stabilized with sodium trifluoromethanesulfonimide, the highest cycling stability of a sodium–air battery was obtained (150 cycles).[17]
Potassium–air
Potassium–air batteries were also proposed with the hopes of overcoming the battery instability associated with superoxide in lithium–air batteries. While only 2-3 charge-discharge cycles have ever been achieved with potassium–air batteries, they do offer an exceptionally low overpotential difference of only 50 mV.[18]
Zinc–air
Magnesium–air
Calcium–air
- No article; see also Calcium: chemical properties for some air (oxygen) reactions.
Aluminum–air
Iron–air
Iron–air rechargeable batteries are an attractive technology with the potential of grid-scale energy storage. The main raw-material of this technology is iron oxide (rust) which is abundant, non-toxic, inexpensive and environmentally friendly.[19] Most of the batteries being developed right now utilize iron oxide (mostly powders) to generate/store hydrogen via the Fe/FeO reduction/oxidation (redox) reaction (Fe + H2O = FeO + H2).[20] In conjunction with a fuel cell this enables the system to behave as a rechargeable battery creating H2O/H2 via production/consumption of electricity.[21] Furthermore, this technology has minimal environmental impact as it could be used to store energy from intermittent solar and wind power sources, developing an energy system with low carbon dioxide emissions.
The way the system works can start by using the Fe/FeO redox reaction, then the hydrogen created during the oxidation of iron can be consumed by a fuel cell in conjunction with oxygen from the air to create electricity. When electricity must be stored, hydrogen generated from water by operating the fuel cell in reverse is consumed during the reduction of the iron oxide to metallic iron.[20][21] The combination of both of this cycles is what makes the system operate as an iron–air rechargeable battery.
Limitations of this technology come from the materials used. Generally, iron oxide powder beds are selected, however, rapid sintering and pulverization of the powders limit the ability to achieve a high number of cycles resulting in a lower capacity. Other methods, such as 3D-Printing[22] and freeze casting,[23][24] currently under investigation seek to enable the creation of architecture materials to allow for high surface area and volume changes during the redox reaction.
Silicon–air
See also
References
- ↑ Metal Air Batteries, Half a Fuel Cell?
- ↑ "METAL-AIR BATTERIES Lithium, Aluminum, Zinc, and Carbon" (PDF). Retrieved 2013-04-04.
- ↑ Li, Y.; Lu, J. (2017). "Metal–Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice?". ACS Energy Letters. 2 (6): 1370–1377. doi:10.1021/acsenergylett.7b00119.
- ↑ Zhang, X.; Wang, X.; Xie, Z.; Zhou, Z. (2016). "Recent progress in rechargeable alkali metal-air batteries". Green Energy & Environment. 1 (1): 1–4. doi:10.1016/j.gee.2016.04.004.
- ↑ "Electrically Rechargeable Metal-Air Batteries (ERMAB)". Retrieved 25 March 2012.
- ↑ "Batteries for Oxygen Concentrators".
- 1 2 "A Low-Overpotential Potassium−Oxygen Battery Based on Potassium Superoxide".
- 1 2 "A Low-Overpotential Potassium−Oxygen Battery Based on Potassium Superoxide".
- ↑ Sun, Qian (2012). "Electrochemical properties of room temperature sodium–air batteries with non-aqueous electrolyte". Electrochemistry Communications. 16: 22–25. doi:10.1016/j.elecom.2011.12.019.
- ↑ "BASF investigating sodium-air batteries as alternative to Li-air; patent application filed with USPTO".
- ↑ Durmus, Y.E.; Aslanbas, O.; Kayser, S.; Tempel, H.; Hausen, F.; de Haart, L.G.J.; Granwehr, J.; Ein-Eli, Y.; Eichel, R.-A.; Kungl, H. (2017). "Long run discharge, performance and efficiency of primary Silicon–air cells with alkaline electrolyte". Electrochimica Acta. 225: 215–224. doi:10.1016/j.electacta.2016.12.120.
- ↑ Ju, HyungKuk; Lee, Jaeyoung (2015). "High-temperature liquid Sn-air energy storage cell". Journal of Energy Chemistry. 24: 614–619. doi:10.1016/j.jechem.2015.08.006.
- ↑ Girishkumar, G.; McCloskey, B.; Luntz, C.; Swanson, S.; Wilcke, W. (2010). "Lithium-Air Battery: Promise and Challenges". The Journal of Physical Chemistry Letters. 1: 2193–2203. doi:10.1021/jz1005384.
- ↑ Kraytsberg, Alexander; Ein-Eli, Yair (2011). "Review on Li–air batteries—Opportunities, limitations and perspective". Journal of Power Sources. 196 (3): 886–893. Bibcode:2011JPS...196..886K. doi:10.1016/j.jpowsour.2010.09.031.
- ↑ Zyga, Lisa. "Sodium-air battery offers rechargeable advantages compared to Li-air batteries". Phys.org. Retrieved 1 March 2018.
- ↑ Hartmann, P.; Bender, C.; Vracar, M.; Durr, A.; Garsuch, A.; Janek, J.; Adelhelm, P. (2012). "A rechargeable room-temperature sodium superoxide (NaO2) battery". Nature Materials Letters. 12 (1): 228–232. Bibcode:2013NatMa..12..228H. doi:10.1038/NMAT3486.
- ↑ He, M.; Lau, K.; Ren, X.; Xiao, N.; McCulloch, W.; Curtiss, L.; Wu, Y. (2016). "Concentrated Electrolyte for the Sodium–Oxygen Battery: Solvation Structure and Improved Cycle Life". Angewandte Chemie. 55 (49): 15310–15314. doi:10.1002/anie.201608607.
- ↑ Ren, X.; Wu, Y. (2013). "A Low-Overpotential Potassium−Oxygen Battery Based on Potassium Superoxide". Journal of the American Chemical Society. 135: 2923−2926. doi:10.1021/ja312059q.
- ↑ Narayanan, S. R.; Prakash, G. K. Surya; Manohar, A.; Yang, Bo; Malkhandi, S.; Kindler, Andrew (2012-05-28). "Materials challenges and technical approaches for realizing inexpensive and robust iron–air batteries for large-scale energy storage". Solid State Ionics. "Fuel Cells-Energy Conversion" Proceedings of Symposium X EMRS Spring Meeting 2011E-MRS / MRS BILATERAL CONFERENCE on ENERGY,"Held at the E-MRS 2011 SPRING MEETING IUMRS ICAM 2011. 216: 105–109. doi:10.1016/j.ssi.2011.12.002.
- 1 2 Requies, J.; Güemez, M. B.; Gil, S. Perez; Barrio, V. L.; Cambra, J. F.; Izquierdo, U.; Arias, P. L. (2013-04-19). "Natural and synthetic iron oxides for hydrogen storage and purification". Journal of Materials Science. 48 (14): 4813–4822. Bibcode:2013JMatS..48.4813R. doi:10.1007/s10853-013-7377-7. ISSN 0022-2461.
- 1 2 Ju, Young-Wan; Ida, Shintaro; Inagaki, Toru; Ishihara, Tatsumi (2011-08-01). "Reoxidation behavior of Ni–Fe bimetallic anode substrate in solid oxide fuel cells using a thin LaGaO3 based film electrolyte". Journal of Power Sources. 196 (15): 6062–6069. Bibcode:2011JPS...196.6062J. doi:10.1016/j.jpowsour.2011.03.086.
- ↑ Jakus, Adam E.; Taylor, Shannon L.; Geisendorfer, Nicholas R.; Dunand, David C.; Shah, Ramille N. (2015-12-01). "Metallic Architectures from 3D-Printed Powder-Based Liquid Inks". Advanced Functional Materials. 25 (45): 6985–6995. doi:10.1002/adfm.201503921. ISSN 1616-3028.
- ↑ Sepúlveda, Ranier; Plunk, Amelia A.; Dunand, David C. (2015-03-01). "Microstructure of Fe2O3 scaffolds created by freeze-casting and sintering". Materials Letters. 142: 56–59. doi:10.1016/j.matlet.2014.11.155.
- ↑ Durán, P.; Lachén, J.; Plou, J.; Sepúlveda, R.; Herguido, J.; Peña, J. A. (2016-11-16). "Behaviour of freeze-casting iron oxide for purifying hydrogen streams by steam-iron process". International Journal of Hydrogen Energy. The 5th Iberian Symposium on Hydrogen, Fuel Cells and Advanced Batteries (HYCELTEC 2015), 5–8 July 2015, Tenerife, Spain. 41 (43): 19518–19524. doi:10.1016/j.ijhydene.2016.06.062.
Notes
- ↑ Calculated from the specific energy density (including oxygen) value and 39.1 and 16 atomic weight data for K and O respectively for KO2