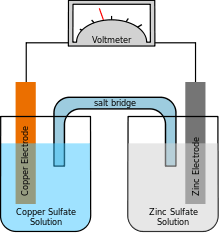
Flow battery
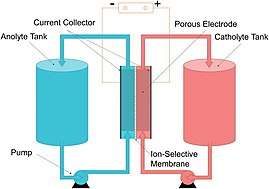
A flow battery, or redox flow battery (after reduction–oxidation), is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids contained within the system and separated by a membrane.[2][3] Ion exchange (accompanied by flow of electric current) occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.2 volts.
A flow battery may be used like a fuel cell (where the spent fuel is extracted and new fuel is added to the system) or like a rechargeable battery (where an electric power source drives regeneration of the fuel). While it has technical advantages over conventional rechargeables, such as potentially separable liquid tanks and near unlimited longevity, current implementations are comparatively less powerful and require more sophisticated electronics.
The energy capacity is a function of the electrolyte volume (amount of liquid electrolyte) and the power a function of the surface area of the electrodes.
Construction principle
A flow battery is a rechargeable fuel cell in which an electrolyte containing one or more dissolved electroactive elements flows through an electrochemical cell that reversibly converts chemical energy directly to electricity (electroactive elements are "elements in solution that can take part in an electrode reaction or that can be adsorbed on the electrode"[4]). Additional electrolyte is stored externally, generally in tanks, and is usually pumped through the cell (or cells) of the reactor, although gravity feed systems are also known.[5] Flow batteries can be rapidly "recharged" by replacing the electrolyte liquid (in a similar way to refilling fuel tanks for internal combustion engines) while simultaneously recovering the spent material for re-energization. Many flow batteries use carbon felt electrodes due to its low cost and adequate electrical conductivity, although these electrodes somewhat limit the charge / discharge power due to their low inherent activity towards many redox couples [6][7].
In other words, a flow battery is just like an electrochemical cell, with the exception that the ionic solution (electrolyte) is not stored in the cell around the electrodes. Rather, the ionic solution is stored outside of the cell, and can be fed into the cell in order to generate electricity. The total amount of electricity that can be generated depends on the size of the storage tanks.
Flow batteries are governed by the design principles established by electrochemical engineering.[8]
Types
Various types of flow cells (batteries) have been developed,[9] including redox, hybrid and membraneless. The fundamental difference between conventional batteries and flow cells is that energy is stored not as the electrode material in conventional batteries but as the electrolyte in flow cells.
Redox
The redox (reduction–oxidation) cell is a reversible cell in which electrochemical components are dissolved in the electrolyte. Redox flow batteries are rechargeable (secondary cells).[10] Because they employ heterogeneous electron transfer rather than solid-state diffusion or intercalation they are more appropriately called fuel cells rather than batteries. In industrial practice, fuel cells are usually, and unnecessarily, considered to be primary cells, such as the H
2/O
2 system. The unitized regenerative fuel cell on NASA's Helios Prototype is another reversible fuel cell. The European Patent Organisation classifies redox flow cells (H01M8/18C4) as a sub-class of regenerative fuel cells (H01M8/18). Examples of redox flow batteries are the Vanadium redox flow battery, polysulfide bromide battery (Regenesys), and uranium redox flow battery.[11] Redox fuel cells are less common commercially although many systems have been proposed.[12][13][14][15]
A prototype zinc-polyiodide flow battery has been demonstrated with an energy density of 167 Wh/l (watt-hours per liter). Older zinc-bromide cells reach 70 Wh/l. For comparison, lithium iron phosphate batteries store 233 Wh/l. The zinc-polyiodide battery is claimed to be safer than other flow batteries given its absence of acidic electrolytes, nonflammability and operating range of −4 to 122 °F (−20 to 50 °C) that does not require extensive cooling circuitry, which would add weight and occupy space. One unresolved issue is zinc build-up on the negative electrode that permeated the membrane, reducing efficiency. Because of the Zn dendrite formation, the Zn-halide batteries cannot operate at high current density (> 20 mA/cm2) and thus have limited power density. Adding alcohol to the electrolyte of the ZnI battery can slightly control the problem.[16]
When the battery is fully discharged, both tanks hold the same electrolyte solution: a mixture of positively charged zinc ions (Zn2+
) and negatively charged iodide ion, I-. When charged, one tank holds another negative ion, polyiodide, I3-. The battery produces power by pumping liquid from external tanks into the battery's stack area where the liquids are mixed. Inside the stack, zinc ions pass through a selective membrane and change into metallic zinc on the stack's negative side.[17] To further increase the energy density of the zinc-iodide flow battery, bromide ions (Br–) are used as the complexing agent to stabilize the free iodine, forming iodine-bromide ions (I2Br–) as a means to free up iodide ions for charge storage.[18]
Traditional flow battery chemistries have both low specific energy (which makes them too heavy for fully electric vehicles) and low specific power (which makes them too expensive for stationary energy storage). However a high power of 1.4 W/cm2 was demonstrated for hydrogen-bromine flow batteries, and a specific energy (530 Wh/kg at the tank level) was shown for hydrogen-bromate flow batteries[19][20][21]
One system uses organic polymers and a saline solution with a cellulose membrane. The prototype withstood 10000 charging cycles while retaining substantial capacity. The energy density was 10 Wh/l.[22] Current density reached 100 milliamperes/cm2.[23]
Hybrid
The hybrid flow battery uses one or more electroactive components deposited as a solid layer.[24] In this case, the electrochemical cell contains one battery electrode and one fuel cell electrode. This type is limited in energy by the surface area of the electrode. Hybrid flow batteries include the zinc-bromine, zinc–cerium[25], lead–acid, and iron-salt flow batteries. Weng et al. [26] reported a Vanadium-Metal hydride rechargeable hybrid flow battery with an experimental OCV of 1.93 V and operating voltage of 1.70 V, very high values among rechargeable flow batteries with aqueous electrolytes. This hybrid battery consists of a graphite felt positive electrode operating in a mixed solution of VOSO4 and H2SO4, and a metal hydride negative electrode in KOH aqueous solution. The two electrolytes of different pH are separated by a bipolar membrane. The system demonstrated good reversibility and high efficiencies in coulomb (95%), energy (84%), and voltage (88%). They reported further improvements of this new redox couple with achievements of increased current density, operation of larger 100 cm2 electrodes, and the operation of 10 large cells in series. Preliminary data using a fluctuating simulated power input tested the viability toward kWh scale storage.[27] Recently, a high energy density Mn(VI)/Mn(VII)-Zn hybrid flow battery has been proposed.[28]
Membraneless
A membraneless battery[29] relies on laminar flow in which two liquids are pumped through a channel. They undergo electrochemical reactions to store or release energy. The solutions stream through in parallel, with little mixing. The flow naturally separates the liquids, eliminating the need for a membrane.[30]
Membranes are often the most costly component and the least reliable components of batteries, as they can corrode with repeated exposure to certain reactants. The absence of a membrane enabled the use of a liquid bromine solution and hydrogen. This combination is problematic when membranes are used, because they form hydrobromic acid that can destroy the membrane. Both materials are available at low cost.[31]
The design uses a small channel between two electrodes. Liquid bromine flows through the channel over a graphite cathode and hydrobromic acid flows under a porous anode. At the same time, hydrogen gas flows across the anode. The chemical reaction can be reversed to recharge the battery—a first for any membraneless design.[31] One such membraneless flow battery published in August 2013 produced a maximum power density of 7950 W/m2, three times as much power as other membraneless systems— and an order of magnitude higher than lithium-ion batteries.[31]
Recently, a macroscale membraneless redox flow battery capable of recharging and recirculation of the same electrolyte streams for multiple cycles has been demonstrated. The battery is based on immiscible organic catholyte and aqueous anolyte liquids, which exhibits high capacity retention and columbic efficiency during cycling.[32]
Primus Power has developed patented technology in its zinc bromine flow battery, a type of redox flow battery, to eliminate the membrane or separator, which reduces costs and failure rates. The Primus Power membraneless redox flow battery is working in installations in the United States and Asia with a second generation product announced 21 February 2017.
Organic
Compared to traditional aqueous inorganic redox flow batteries such as vanadium redox flow batteries and Zn-Br2 batteries, which have been developed for decades, organic redox flow batteries emerged in 2009 and hold great promise to overcome major drawbacks preventing economical and extensive deployment of traditional inorganic redox flow batteries. The primary merit of organic redox flow batteries lies in the tunable redox properties of the redox-active components.
Organic redox flow batteries could be further classified into two categories: Aqueous Organic Redox Flow Batteries (AORFBs) and Non-aqueous Organic Redox Flow Batteries (NAORFBs)[33][34][35][36]. AORFBs use water as solvent for electrolyte materials while NAORFBs employ organic solvents to dissolve redox active materials. Depending on using one or two organic redox active electrolytes as anode and/or cathode, AORFBs and NAORFBs can be further divided into total organic systems and hybrid organic systems that use inorganic materials for anode or cathode. The proof of concept of AORFBs was demonstrated before NAORFBs. In larger-scale energy storage, AORFBs hold much greater potential than NAORFBs because of the former's lower cost, higher current and power performance, as well as safety advantages of aqueous over non-aqueous electrolytes. NAORFBs may find their place in limited special applications for their higher energy density than AORFBs though more challenges are to be overcome in terms of safety, cost of organic solvents, radical induced side reactions, electrolyte crossover and limited life time. The content below mainly covers the representative studies on AORFBs.
Quinones are the basis of some AORFBs.[37][38] In one study, 1,2-dihydrobenzoquinone-3,5-disulfonic acid (BQDS) and 1,4-dihydrobenzoquinone-2-sulfonic acid (BQS) were employed as cathodes, and conventional Pb/PbSO4 was the anolyte in an acid AORFB. These first AORFBs are hybrid systems as they use organic redox active materials only for the cathode side. The quinones accepts two units of electrical charge, compared with one in conventional catholyte, implying that such a battery could store twice as much energy in a given volume.
9,10-Anthraquinone-2,7-disulphonic acid (AQDS), also a quinone, has been evaluated as well.[39] AQDS undergoes rapid, reversible two-electron/two-proton reduction on a glassy carbon electrode in sulphuric acid. An aqueous flow battery with inexpensive carbon electrodes, combining the quinone/hydroquinone couple with the Br
2/Br−
redox couple, yields a peak galvanic power density exceeding 6,000 W/m2 at 13,000 A/m2. Cycling showed >99 % storage capacity retention per cycle. Volumetric energy density was over 20 Wh/l.[40] Anthraquinone-2-sulfonic acid and anthraquinone-2,6-disulfonic acid on the negative side and 1,2-dihydrobenzoquinone- 3,5-disulfonic acid on the positive side avoids the use of hazardous Br2. The battery was claimed to last for 1,000 cycles without degradation although no official data were published.[41] While this total organic system appears robust, it has a low cell voltage (ca. 0.55 V) and a low energy density (< 4 Wh/L).
Hydrobromic acid used as an electrolyte has been replaced with a far less toxic alkaline solution (1M KOH) and ferrocyanide.[42] The higher pH is less corrosive, allowing the use of inexpensive polymer tanks. The increased electrical resistance in the membrane was compensated by increasing the voltage. The cell voltage was 1.2 V.[43][44] The cell's efficiency exceeded 99%, while round-trip efficiency measured 84%. The battery has an expected lifetime of at least 1,000 cycles. Its theoretic energy density was 19 Wh per liter.[45] Ferrocyanide's chemically stability in high pH KOH solution without forming Fe(OH)2 or Fe(OH)3 needs to be verified before scale-up.
Another organic AORFB has been demonstrated methyl viologen as anolyte and 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl as catholyte, plus sodium chloride and a low-cost anion exchange membrane to enable charging and discharging. This MV/TEMPO system has the highest cell voltage, 1.25 V, and, possibly, lowest capital cost ($180/kWh) reported for AORFBs. The water-based liquid electrolytes were designed as a drop-in replacement for current systems without replacing existing infrastructure. A 600-milliwatt test battery was stable for 100 cycles with nearly 100 percent efficiency at current densities ranging from 20 to 100 mA per square centimeter, with optimal performance rated at 40-50 mA, at which about 70 percent of the battery's original voltage was retained.[46][47] The significance of the research is that neutral AORFBs can be more environmentally friendly than acid or alkaline AORFBs while showing electrochemical performance comparable to corrosive acidic or alkaline RFBs. The MV/TEMPO AORFB has an energy density of 8.4 Wh/L with the limitation on the TEMPO side. The next step is to identify a high capacity catholyte to match MV (ca. 3.5 M solubility in water, 93.8 Ah/L).
One flow-battery concept is based on redox active, organic polymers employs viologen and TEMPO with dialysis membranes. The polymer-based redox-flow battery (pRFB) uses functionalized macromolecules (similar to acrylic glass or Styrofoam) being dissolved in water as active material for the anode as well as the cathode. Thereby, metals and strongly corrosive electrolytes – like vanadium salts in sulfuric acid – are avoided and simple dialysis membranes can be employed. The membrane, which separates the cathode and the anode of the flow cell, works like a strainer and is produced much more easily and at lower cost than conventional ion-selective membranes. It retains the big “spaghetti”-like polymer molecules, while allowing the small counterions to pass.[48] The concept may solve the high cost of traditional Nafion membrane but the design and synthesis of redox active polymer with high solubility in water is not trivial.
Metal hydride
Proton flow batteries (PFB) integrate a metal hydride storage electrode into a reversible proton exchange membrane (PEM) fuel cell. During charging, PFB combines hydrogen ions produced from splitting water with electrons and metal particles in one electrode of a fuel cell. The energy is stored in the form of a solid-state metal hydride. Discharge produces electricity and water when the process is reversed and the protons are combined with ambient oxygen. Metals less expensive than lithium can be used and provide greater energy density than lithium cells.[49][50]
Nano-network
Lithium–sulfur system arranged in a network of nanoparticles eliminates the requirement that charge moves in and out of particles that are in direct contact with a conducting plate. Instead, the nanoparticle network allows electricity to flow throughout the liquid. This allows more energy to be extracted.[51]
Semi-solid
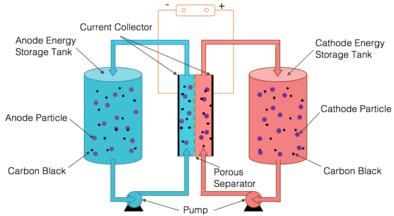
In a semi-solid flow cell, the positive and negative electrodes are composed of particles suspended in a carrier liquid. The positive and negative suspensions are stored in separate tanks and pumped through separate pipes into a stack of adjacent reaction chambers, where they are separated by a barrier such as a thin, porous membrane. The approach combines the basic structure of aqueous-flow batteries, which use electrode material suspended in a liquid electrolyte, with the chemistry of lithium-ion batteries in both carbon-free suspensions and slurries with conductive carbon network.[53][54][55] The carbon free semi-solid redox flow battery is also sometimes referred to as Solid Dispersion Redox Flow Battery.[56] Dissolving a material changes its chemical behavior significantly. However, suspending bits of solid material preserves the solid's characteristics. The result is a viscous suspension that flows like molasses.[57]
Chemistries
A wide range of chemistries have been tried for flow batteries.[2]
| Couple | Max. cell voltage (V) | Average electrode power density (W/m2) | Average fluid energy density (W·h/kg or W·h/L) | cycles |
|---|---|---|---|---|
| Hydrogen–lithium bromate | 1.1 | 15,000 | 750 Wh/Kg | |
| Hydrogen–lithium chlorate | 1.4 | 10,000 | 1400 Wh/Kg | |
| Bromine-hydrogen | 1.07 | 7,950 | ||
| Iron–tin | 0.62 | <200 | ||
| Iron–titanium | 0.43 | <200 | ||
| Iron–chrome | 1.07 | <200 | ||
| Organic (2013) | 0.8 | 13000 | 21.4 Wh/L | 10 |
| Organic (2015) | 1.2 | 7.1 Wh/L | 100 | |
| MV-TEMPO | 1.25 | 8.4 Wh/L | 100 | |
| Vanadium–vanadium (sulphate) | 1.4 | ~800 | 25 Wh/L | |
| Vanadium–vanadium (bromide) | 50 Wh/L | 2000[2] | ||
| Sodium–bromine polysulfide | 1.54 | ~800 | ||
| Zinc–bromine | 1.85 | ~1,000 | 75 Wh/Kg | |
| Lead–acid (methanesulfonate) | 1.82 | ~1,000 | ||
| Zinc–cerium (methanesulfonate) | 2.43 | <1,200–2,500 | ||
| Zn-Mn(VI)/Mn(VII) | 1.2 | 60 Wh/L[28] |
Advantages and disadvantages
Redox flow batteries, and to a lesser extent hybrid flow batteries, have the advantages of flexible layout (due to separation of the power and energy components), long cycle life (because there are no solid-to-solid phase transitions), quick response times, no need for "equalisation" charging (the overcharging of a battery to ensure all cells have an equal charge) and no harmful emissions. Some types also offer easy state-of-charge determination (through voltage dependence on charge), low maintenance and tolerance to overcharge/overdischarge. Compared to solid-state rechargeable batteries such as Li ion, RFBs, and ARFBs in particular, can operate at much higher current and power densities. These technical merits make redox flow batteries a well-suited option for large-scale energy storage.
On the negative side, the energy densities vary considerably but are, in general, lower compared to portable batteries, such as the Li-ion.
Also, compared to nonreversible fuel cells or electrolyzers using similar electrolytic chemistries, flow batteries generally have somewhat lower efficiency.
The development and economy in going from laboratory to industry-scale is an ongoing process. Component cost is one of several aspects of this process. A sulfur-oxygen-salt chemistry has been demonstrated in laboratory.[58]
Applications
Flow batteries are normally considered for relatively large (1 kWh – 10 MWh) stationary applications. These are for
- Load balancing – where the battery is connected to an electrical grid to store excess electrical power during off-peak hours and release electrical power during peak demand periods. The common problem limiting the use of most flow battery chemistries in this application is their low areal power (operating current density) which translates into a high cost of power.
- Storing energy from renewable sources such as wind or solar for discharge during periods of peak demand.[59]
- Peak shaving, where spikes of demand are met by the battery.[60]
- UPS, where the battery is used if the main power fails to provide an uninterrupted supply.
- Power conversion – because all cells share the same electrolyte/s. Therefore, the electrolyte/s may be charged using a given number of cells and discharged with a different number. Because the voltage of the battery is proportional to the number of cells used the battery can therefore act as a very powerful DC–DC converter. In addition, if the number of cells is continuously changed (on the input and/or output side) power conversion can also be AC/DC, AC/AC, or DC–AC with the frequency limited by that of the switching gear.[61]
- Electric vehicles – Because flow batteries can be rapidly "recharged" by replacing the electrolyte, they can be used for applications where the vehicle needs to take on energy as fast as a combustion engined vehicle.[62][63] A common problem found with most RFB chemistries in the EV applications is their low energy density which translated into a short driving range. Flow batteries based on highly soluble halates are a notable exception.[64]
- Stand-alone power system – An example of this is in cellphone base stations where no grid power is available. The battery can be used alongside solar or wind power sources to compensate for their fluctuating power levels and alongside a generator to make the most efficient use of it to save fuel.[65][66] Currently, flow batteries are being used in solar micro grid applications throughout the Caribbean.
See also
References
- ↑ Qi, Zhaoxiang; Koenig, Gary M. (2017-05-12). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena. 35 (4): 040801. doi:10.1116/1.4983210. ISSN 2166-2746.
- 1 2 3 Badwal, Sukhvinder P. S.; Giddey, Sarbjit S.; Munnings, Christopher; Bhatt, Anand I.; Hollenkamp, Anthony F. (24 September 2014). "Emerging electrochemical energy conversion and storage technologies". Frontiers in Chemistry. 2. doi:10.3389/fchem.2014.00079. PMC 4174133. PMID 25309898.
- ↑ Alotto, P.; Guarnieri, M.; Moro, F. (2014). "Redox Flow Batteries for the storage of renewable energy: a review". Renewable & Sustainable Energy Reviews. 29: 325–335. doi:10.1016/j.rser.2013.08.001.
- ↑ Science-Dictionary.org. "Electroactive Substance" 14 May 2013.
- ↑ T. Fujii, T. Hirose, and N. Kondou, in JP patent 55096569 (1979), to Meidensha Electric Mfg. Co. Ltd.
- ↑ Aaron, Douglas. "In Situ Kinetics Studies in All-Vanadium Redox Flow Batteries". ECS Electrochemistry Letters. 2 (3): A29. doi:10.1149/2.001303eel. More than one of
|pages=and|page=specified (help) - ↑ McCreery, Richard L. (2008-07). "Advanced Carbon Electrode Materials for Molecular Electrochemistry". Chemical Reviews. 108 (7): 2646–2687. doi:10.1021/cr068076m. ISSN 0009-2665. Check date values in:
|date=(help) - ↑ Arenas, L.F.; Ponce de León, C.; Walsh, F.C. (June 2017). "Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage". Journal of Energy Storage. 11: 119–153. doi:10.1016/j.est.2017.02.007.
- ↑ Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. (2015). "The Chemistry of Redox-Flow Batteries". Angew. Chem. Int. Ed. 54: 9776–9809. doi:10.1002/anie.201410823.
- ↑ Linden, D.; Reddy, T.B. (2002). Handbook of Batteries (Eds.). McGraw-Hill.
- ↑ Shiokawa, Y.; Yamana, H.; Moriyama, H. (2000). "An Application of Actinide Elements for a Redox Flow Battery". Journal of Nuclear Science and Technology. 37 (3): 253–256. doi:10.1080/18811248.2000.9714891.
- ↑ W. Borchers, in US patent 567959 (1894)
- ↑ W. Nernst, in DE patent 264026 (1912)
- ↑ R. M. Keefer, in US patent 3682704 (1970), to Electrocell Ltd.
- ↑ Kummer, J. T.; Oei, D. -G. (1985). "A chemically regenerative redox fuel cell. II". Journal of Applied Electrochemistry. 15 (4): 619–629. doi:10.1007/BF01059304.
- ↑ Borghino, Dario (27 February 2015). "High-performance flow battery could rival lithium-ions for EVs and grid storage". Gizmag.
- ↑ White, Frances (25 February 2015). "New flow battery to keep big cities lit, green and safe". R&D.
- ↑ Weng, Guo-Ming (2017). "Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries". Energy & Environmental Science. 3: 735–741. doi:10.1039/C6EE03554J.
- ↑ "Cyclic Performance Analysis of Hydrogen/Bromine Flow Batteries for Grid-Scale Energy Storage". doi:10.1002/cplu.201402043/suppinfo.
- ↑ Yu; Tolmachev, V. (2013). "Hydrogen-halogen electrochemical cells: A review of applications and technologies". Russian Journal of Electrochemistry. 50: 301–316. doi:10.1134/S1023193513120069.
- ↑ Tolmachev, Yuriy V. (2015). "Energy cycle based on a high specific energy aqueous flow battery and its potential use for fully electric vehicles and for direct solar-to-chemical energy conversion". Journal of Solid State Electrochemistry. 19: 2711–2722. doi:10.1007/s10008-015-2805-z.
- ↑ "Chemists present an innovative redox-flow battery based on organic polymers and water". phys.org. Phys.org. 21 October 2015. Retrieved 6 December 2015.
- ↑ Janoschka, Tobias; Martin, Norbert; Martin, Udo; Friebe, Christian; Morgenstern, Sabine; Hiller, Hannes; Hager, Martin D.; Schubert, Ulrich S. (2015). "An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials". Nature. 527 (7576): 78–81. doi:10.1038/nature15746. PMID 26503039.
- ↑ Bartolozzi, M. (1989). "Development of redox flow batteries. A historical bibliography". Journal of Power Sources. 27 (3): 219–234. doi:10.1016/0378-7753(89)80037-0.
- ↑ Leung, P. K.; Ponce-De-León, C.; Low, C. T. J.; Shah, A. A.; Walsh, F. C. (2011). "Characterization of a zinc–cerium flow battery". Journal of Power Sources. 196 (11): 5174–5185. doi:10.1016/j.jpowsour.2011.01.095.
- ↑ J. Electrochem. Soc. 2013 volume 160, issue 9, A1384-A1389
- ↑ J. Electrochem. Soc. 2016 volume 163, issue 1, A5180-A5187
- 1 2 Chem. Commun. 52 (2016) 14039-14042. DOI:10.1039/C6CC08070G
- ↑ Bamgbopa, Musbaudeen O.; Almheiri, Saif; Sun, Hong (2017-04). "Prospects of recently developed membraneless cell designs for redox flow batteries". Renewable and Sustainable Energy Reviews. 70: 506–518. doi:10.1016/j.rser.2016.11.234. ISSN 1364-0321. Check date values in:
|date=(help) - ↑ "New rechargeable flow battery enables cheaper, large-scale energy storage". KurzweilAI. doi:10.1038/ncomms3346. Retrieved 20 August 2013.
- 1 2 3 Braff, W. A.; Bazant, M. Z.; Buie, C. R. (2013). "Membrane-less hydrogen bromine flow battery". Nature Communications. 4: 2346. doi:10.1038/ncomms3346. PMID 23949161.
- ↑ Bamgbopa, Musbaudeen O.; Shao-Horn, Yang; Hashaikeh, Raed; Almheiri, Saif (2018-03). "Cyclable membraneless redox flow batteries based on immiscible liquid electrolytes: Demonstration with all-iron redox chemistry". Electrochimica Acta. 267: 41–50. doi:10.1016/j.electacta.2018.02.063. ISSN 0013-4686. Check date values in:
|date=(help) - ↑ Bamgbopa, Musbaudeen O.; Shao-Horn, Yang; Almheiri, Saif (2017). "The potential of non-aqueous redox flow batteries as fast-charging capable energy storage solutions: demonstration with an iron–chromium acetylacetonate chemistry". Journal of Materials Chemistry A. 5 (26): 13457–13468. doi:10.1039/c7ta02022h. ISSN 2050-7488.
- ↑ Bamgbopa, Musbaudeen O.; Almheiri, Saif (2017-02). "Influence of solvents on species crossover and capacity decay in non-aqueous vanadium redox flow batteries: Characterization of acetonitrile and 1, 3 dioxolane solvent mixture". Journal of Power Sources. 342: 371–381. doi:10.1016/j.jpowsour.2016.12.050. ISSN 0378-7753. Check date values in:
|date=(help) - ↑ Bamgbopa, Musbaudeen O.; Pour, Nir; Shao-Horn, Yang; Almheiri, Saif (2017-01). "Systematic selection of solvent mixtures for non-aqueous redox flow batteries – vanadium acetylacetonate as a model system". Electrochimica Acta. 223: 115–123. doi:10.1016/j.electacta.2016.12.014. ISSN 0013-4686. Check date values in:
|date=(help) - ↑ Mustafa, Ibrahim; Bamgbopa, Musbaudeen O.; Alraeesi, Eman; Shao-Horn, Yang; Sun, Hong; Almheiri, Saif (2017-01-01). "Insights on the Electrochemical Activity of Porous Carbonaceous Electrodes in Non-Aqueous Vanadium Redox Flow Batteries". Journal of The Electrochemical Society. 164 (14): A3673–A3683. doi:10.1149/2.0621714jes. ISSN 0013-4651.
- ↑ Xu, Y.; Wen, Y.; Cheng, J.; Yanga, Y.; Xie, Z.; Cao, G. In World Non-Grid-Connected Wind Power and Energy Conference, 2009. WNWEC 2009 IEEE: Nanjing, China, 2009, p 1.
- ↑ Xu, Yan; Wen, Yue-Hua; Cheng, Jie; Cao, Gao-Ping; Yang, Yu-Sheng (2010). "A study of tiron in aqueous solutions for redox flow battery application". Electrochimica Acta. 55 (3): 715–720. doi:10.1016/j.electacta.2009.09.031. ISSN 0013-4686.
- ↑ WALD, MATTHEW L. (8 January 2014). "From Harvard, a Cheaper Storage Battery". New York Times. Retrieved 10 January 2014.
- ↑ "Harvard team demonstrates new metal-free organic–inorganic aqueous flow battery; potential breakthrough for low-cost grid-scale storage". 11 January 2014.
- ↑ Szondy, David (29 June 2014). "New water-based organic battery is cheap, rechargeable and eco-friendly". Gizmag.
- ↑ "A rechargeable battery to power a home from rooftop solar panels".
- ↑ Matthew Gunther,ChemistryWorld. "Flow Battery Could Smooth Irregular Wind and Solar Energy Supply". Scientific American.
- ↑ Alkaline quinone flow battery Lin et al. Science 2015 349 (6255), p. 1529
- ↑ Borghino, Dario (30 September 2015). "Greener, safer flow battery could store renewable energy on the cheap". www.gizmag.com. Retrieved 8 December 2015.
- ↑ Moss, Richard (22 December 2015). "New flow battery projected to cost 60% less than existing standard". www.gizmag.com. Retrieved 23 December 2015.
- ↑ Liu, Tianbiao; Wei, Xiaoliang; Nie, Zimin; Sprenkle, Vincent; Wang, Wei (1 November 2015). "A Total Organic Aqueous Redox Flow Battery Employing a Low Cost and Sustainable Methyl Viologen Anolyte and 4-HO-TEMPO Catholyte". Advanced Energy Materials. 6: 1501449. doi:10.1002/aenm.201501449. ISSN 1614-6840.
- ↑ Tobias Janoschka, Norbert Martin, Udo Martin, Christian Friebe, Sabine Morgenstern, Hannes Hiller, Martin D. Hager, Ulrich S. Schubert (2015). "An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials". Nature. doi:10.1038/nature15746
- ↑ "Proton flow battery simplifies hydrogen power". Gizmag.com. Retrieved 13 February 2014.
- ↑ Andrews, J.; Seif Mohammadi, S. (2014). "Towards a 'proton flow battery': Investigation of a reversible PEM fuel cell with integrated metal-hydride hydrogen storage". International Journal of Hydrogen Energy. 39 (4): 1740–1751. doi:10.1016/j.ijhydene.2013.11.010.
- ↑ Kevin Bullis (24 April 2014). "Nanoparticle Networks Promise Cheaper Batteries for Storing Renewable Energy". MIT Technology Review. Retrieved 24 September 2014.
- ↑ Qi, Zhaoxiang; Koenig, Gary M. (2017-07). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena. 35 (4): 040801. doi:10.1116/1.4983210. ISSN 2166-2746. Check date values in:
|date=(help) - ↑ Qi, Zhaoxiang; Koenig, Gary M. (2017-05-12). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena. 35 (4): 040801. doi:10.1116/1.4983210. ISSN 2166-2746.
- ↑ Duduta, Mihai (May 2011). "Semi-Solid Lithium Rechargeable Flow Battery". Advanced Energy Materials. 1: 511–516. doi:10.1002/aenm.201100152.
- ↑ Qi, Zhaoxiang; Koenig Jr., Gary M. (2016-08-15). "A carbon-free lithium-ion solid dispersion redox couple with low viscosity for redox flow batteries". Journal of Power Sources. 323: 97–106. doi:10.1016/j.jpowsour.2016.05.033.
- ↑ Qi, Zhaoxiang; Liu, Aaron L.; Koenig Jr, Gary M. (2017-02-20). "Carbon-free Solid Dispersion LiCoO2 Redox Couple Characterization and Electrochemical Evaluation for All Solid Dispersion Redox Flow Batteries". Electrochimica Acta. 228: 91–99. doi:10.1016/j.electacta.2017.01.061.
- ↑ Chandler, David L. (23 August 2011). "Go with the Flow - Cambridge Crude". Technology Review.
- ↑ Li, Zheng; Sam Pan, Menghsuan; Su, Liang; Tsai, Ping-Chun; Badel, Andres F.; Valle, Joseph M.; Eiler, Stephanie L.; Xiang, Kai; Brushett, Fikile R.; Chiang, Yet-Ming (2017-10-11). "Air-Breathing Aqueous Sulfur Flow Battery for Ultralow-Cost Long-Duration Electrical Storage". Joule. 1: 306–327. doi:10.1016/j.joule.2017.08.007.
- ↑ REDT Energy. "Storing Renewable Energy".
- ↑ Archived 9 February 2010 at the Wayback Machine.
- ↑ P. M. Spaziante, K. Kampanatsanyakorn, and A. Zocchi, in WO patent 03043170 (2001), to Squirrel Holdings Ltd.
- ↑ "Electric Vehicle Refuelling System (EVRS) used in conjunction with Vanadium Redox Flow Technology". REDT Energy Storage.
- ↑ Antony Ingram. "nanoFLOWCELL-powered Quant e-Limo approved for german road trials". Fox News.
- ↑ Tolmachev, Yuriy V.; Piatkivskyi, Andrii; Ryzhov, Victor V.; Konev, Dmitry V.; Vorotyntsev, Mikhail A. (2015). "Energy cycle based on a high specific energy aqueous flow battery and its potential use for fully electric vehicles and for direct solar-to-chemical energy conversion". Journal of Solid State Electrochemistry. 19 (9): 2711–2722. doi:10.1007/s10008-015-2805-z.
- ↑ Talk by John Davis of Deeya energy about their flow battery's use in the telecomms industry on YouTube
- ↑ Performance Testing of Zinc-Bromine Flow Batteries for Remote Telecom Sites